Popular on s4story
- New Book "Downsize With Dignity" Helps Missouri Families Navigate Senior Moves - 210
- This Christmas 2025, Virginia Veterans Can Make Their Book For Free
- "Has Your Book Been Suppressed?" Widespread Censorship by Amazon, Google, and Meta
- 4-Hour Work Day: Jon Robert Quinn Challenges Hustle Culture and Redefines Entrepreneurial Success
- Impact & Influence Magazine Surpasses 40,000 Subscribers Nationwide
- RNHA Affirms Support for President Trump as Nation Marks Historic Victory for Freedom
- Phillip E Walker's EntryLevelActing.com Actor Employment Advice E-Book Road Map Launches on MLK Day
- UK Financial Ltd Executes Compliance Tasks Ahead Of First-Ever ERC-3643 Exchange-Traded Token, SMCAT & Sets Date For Online Investor Governance Vote
- Guests Can Save 25 Percent Off Last Minute Bookings at KeysCaribbean's Village at Hawks Cay Villas
Similar on s4story
- Daniel Kaufman Launches a Vertically Integrated Real Estate and Investment Platform
- Impact Futures Group expands through acquisition of specialist healthcare sector training provider Caring for Care
- Powering the AI, Defense and Aerospace Future with Energy Infrastructure and Digital Asset Strength: KULR Technology Group, Inc. $KULR
- $10 Price Target in Think Equity Report Supported by Inventory Financing Floorplan Boot to $60 Million for 2026 Sales Growth in Pre-Owned Boats: $OTH
- Acuvance Acquires ROI Healthcare Solutions, Building a Dedicated Healthcare ERP Practice
- Home Prices Just Hit 5X Median Income — So Americans Are Buying Businesses Instead of Houses
- CCHR White Paper Urges Government Crackdown on Troubled Teen and For-Profit Psychiatric Facilities
- Still Searching for the Perfect Valentine's Gift? Lick Personal Oils Offers Romantic, Experience-Driven Alternatives to Traditional Presents
- UK Financial Ltd Announces CoinMarketCap Supply Verification And Market Positioning Review For Regulated Security Tokens SMPRA And SMCAT
- Automation, innovation in healthcare processes featured at international conference in Atlanta
Revenue Expansion, Regulatory Momentum, and a Leadership Position in the $750 Million Suicidal Depression: NRx Pharmaceuticals (N A S D A Q: NRXP)
S For Story/10677849
$NRXP Has Manufactured Multiple Commercial Lots of NRX-100 and KETAFREE™ with a Shelf Life of Three Years.
MIAMI - s4story -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is rapidly emerging as one of the most compelling growth stories in mental-health therapeutics. With three revenue-generating treatment facilities now operating in Florida—and six expected by year-end—the company is entering 2026 with accelerating clinical operations, expanding market share, and advancing two FDA-directed regulatory pathways for ketamine-based therapeutics aimed at suicidal depression, one of the largest unmet needs in mental health.
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
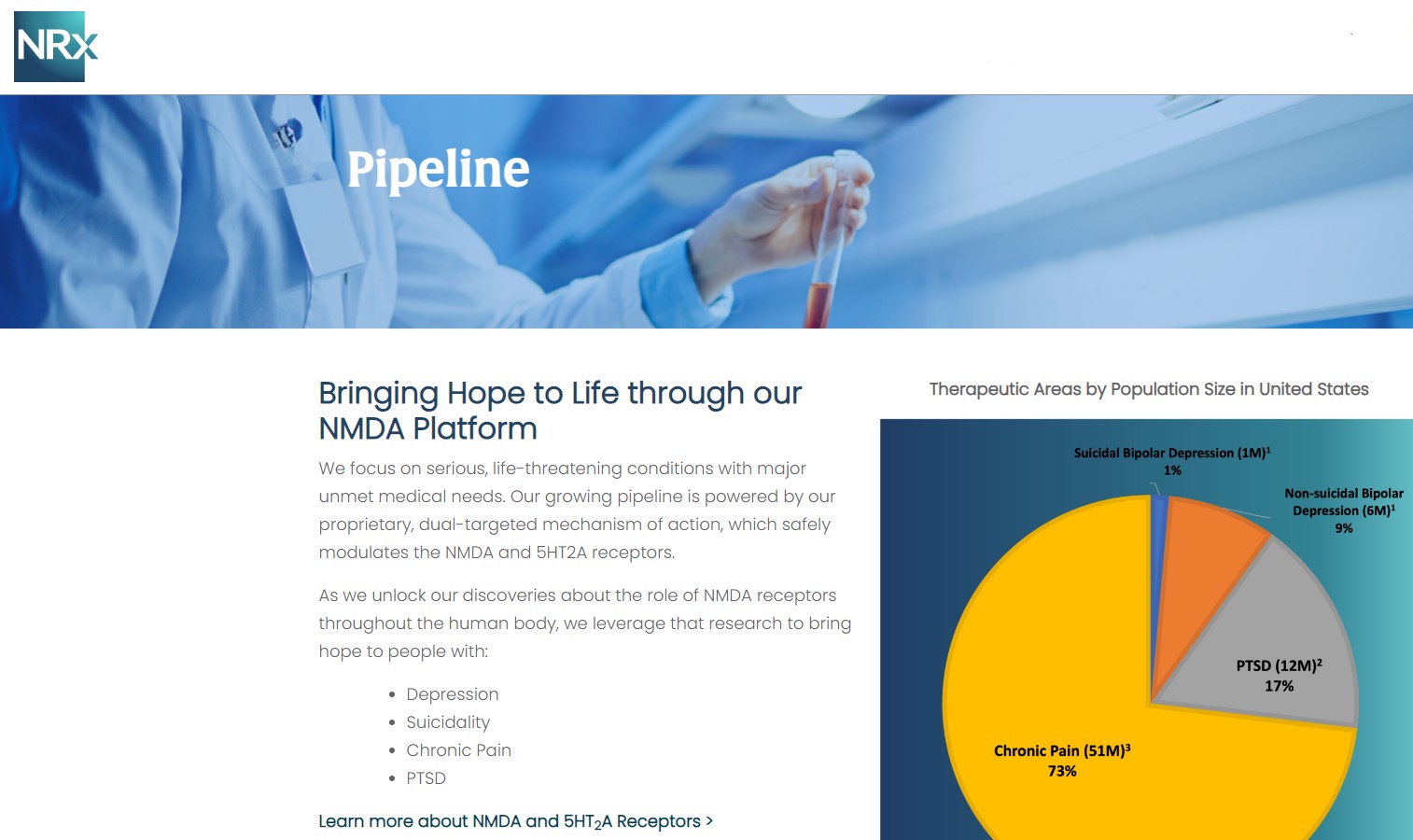
A Mission Targeting One of America's Most Pressing Health Crises
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on S For Story
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
2. ANDA for KETAFREE™ (Generic Ketamine)
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
These facilities support:
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
ONE-D combines:
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on S For Story
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Backed by strong clinical data, FDA Fast Track designations, and third-party revenue projections, NRXP appears positioned to capitalize on a rapidly expanding U.S. ketamine market currently estimated at $750 million, projected to reach $3.35 billion globally by 2034.
Adding to investor enthusiasm, analyst D. Boral issued a Buy rating and a $34 price target, citing NRXP's accelerating execution and diversified commercialization strategy.
A Mission Targeting One of America's Most Pressing Health Crises
More than 13 million Americans seriously consider suicide each year (CDC). NRXP is developing next-generation therapeutics aimed directly at this crisis through its NMDA-based drug platform:
Key Pipeline Assets
- NRX-100 (IV Ketamine):
- FDA Fast Track designation for suicidal ideation in depression, including bipolar depression.
- Multiple commercial lots produced with three-year shelf life stability.
- NDA submission expected in Q4 2025, supported by real-world patient data from more than 60,000 IV ketamine cases.
- KETAFREE™ (preservative-free ketamine):
- Pursuing approval via an ANDA (generic) pathway.
- Recently received supportive FDA correspondence confirming no major deficiencies and on track for a Q2 2026 GDUFA date.
- Addresses a ketamine market worth ~$750 million annually.
- NRX-101 (D-cycloserine + lurasidone):
- FDA-designated Investigational Breakthrough Therapy.
- Targets suicidal treatment-resistant bipolar depression, chronic pain, and potential utility in complicated UTIs.
- New real-world data suggest its active ingredient doubles the effectiveness of TMS—a potential major indication expansion.
NRXP is partnered with Alvogen Pharmaceuticals for the development and commercialization of NRX-101.
More on S For Story
- New Memoir By El Falcon Rojo Run Like a Rarámuri Celebrates Indigenous Wisdom, Endurance and Giving
- Kliemann Brothers Announces 2025 Furnace Giveaway Winners
- Attention Everyone in the Art World: Here is a painting that asks the Quintessential Question about Art Prices for every living artist in the Universe
- Daniel Kaufman Launches a Vertically Integrated Real Estate and Investment Platform
- Michelle Carey Returns to Fiction with Gripping Environmental Thriller "Haze"
A Dual-Path Strategy for NRX-100: Innovative and Generic Market Entry
NRXP's regulatory strategy is unique: it is simultaneously pursuing both an innovative NDA pathway and a generic ANDA pathway for NRX-100 and KETAFREE™.
This creates two powerful value drivers:
1. Innovative NDA for Suicidal Depression
- Expected completion: Q4 2025.
- Includes comparative real-world data showing IV ketamine may have faster onset and greater effect than intranasal S-ketamine.
- NRXP has applied for a Commissioner's National Priority Voucher, which could accelerate FDA review.
2. ANDA for KETAFREE™ (Generic Ketamine)
- Re-filed after FDA approved NRXP's Suitability Petition for its preservative-free formulation.
- Supportive FDA feedback in November 2025 indicated no significant deficiencies.
- A Citizen Petition filed to remove toxic preservative benzethonium chloride from ketamine products could reshape the competitive landscape.
With commercial lots already manufactured, NRXP is positioned for a swift commercial rollout upon approval.
HOPE Clinics: Expanding Revenue Footprint Across Florida
NRXP's HOPE Therapeutics subsidiary is advancing a scalable clinic model for ketamine and advanced TMS-based interventions.
Current & Expected Expansion
- 3 operational facilities in Florida
- 6 additional facilities planned by year-end
These facilities support:
- NRX-100 Expanded Access Program
- Ketamine-based clinical services
- ONE-D single-day TMS depression treatments
- Veterans, first responders, and active-duty military populations
Management expects increased revenue contributions from clinical operations through 2026.
Breakthrough ONE-D: First-in-Florida Deployment with Ampa Health
In November, NRXP launched patient treatment using Ampa Health's ONE-D protocol—a groundbreaking approach reporting:
- 87% response rate
- 72% remission
- Achieved in one day, instead of traditional 90-day TMS schedules
ONE-D combines:
- Ampa's FDA-cleared TMS device
- D-cycloserine (NRX-101 active ingredient)
- Lisdexamfetamine
ONE-D is being deployed at HOPE clinics in Sarasota, Naples, and Fort Myers, with additional locations coming online in 2025.
More on S For Story
- Long Long Tales: Bilingual Cartoon Series on Youtube Celebrating Chinese New Year
- MAX Illumination Redefines Cabinet Displays with New Edge-Lit LED Technology
- Impact Futures Group expands through acquisition of specialist healthcare sector training provider Caring for Care
- FeedSocially - Post Once, Publish Everywhere
- Finland's New Gambling Watchdog Handed Sweeping Powers to Revoke Licenses and Block Illegal Casino Sites
This marks a major competitive differentiator for NRXP in the mental-health treatment sector.
Financial Position: Capital Secured Through July 2026
NRXP reported securing sufficient operating capital to support its drug development programs through July 2026, complementing increasing clinical revenues.
Third-quarter results (released November 17, 2025) highlighted:
- Progress across all clinical and regulatory objectives
- Expanded Fast Track designation for NRX-100
- EU and NIH data enhancing regulatory strategy
- Growth of the HOPE facility platform
- Enhanced IP and formulation positioning
The full Q3 update and webcast are available on the NRXP investor relations site.
High-Value Licensing Opportunity
NRXP has accepted non-binding potential terms from an international pharmaceutical partner for the licensing and distribution of NRX-100. Terms include:
- Over $300 million in milestone payments
- Tiered double-digit royalties
If finalized, this could represent a transformative revenue opportunity for the company.
Investment Outlook
With:
- Multiple clinical catalysts approaching
- Two regulatory pathways advancing for NRX-100
- FDA Breakthrough Therapy designations
- National leadership in cutting-edge TMS + D-cycloserine therapy
- Increasing real-world data support
- A growing network of revenue-producing treatment centers
- Newly manufactured commercial drug supply
- And a strong capital runway
NRx Pharmaceuticals is attracting increased attention from both institutional and retail investors.
The recent $34 price target from D. Boral underscores growing confidence in NRXP's ability to execute on a scalable, high-margin strategy targeting one of the most underserved and urgent areas of mental health.
For More Information
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Chief Business Officer: Matthew Duffy
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on S For Story
- CCHR White Paper Urges Government Crackdown on Troubled Teen and For-Profit Psychiatric Facilities
- Still Searching for the Perfect Valentine's Gift? Lick Personal Oils Offers Romantic, Experience-Driven Alternatives to Traditional Presents
- Boston Industrial Solutions' BPA Certified BX Series Raises the Bar for Pad Printing Inks
- Political Analyst Earl Ofari Hutchinson Charges Good Not the First or Worst ICE Shooting
- Boston Corporate Coach™ Sets Global Standard for Executive Chauffeur Services Across 680 Cities
- Wrathenville Unleashes a Gothic Horror Mystery of Blood, Folklore, and Fate
- UK Financial Ltd Announces CoinMarketCap Supply Verification And Market Positioning Review For Regulated Security Tokens SMPRA And SMCAT
- Sharpe Automotive Redefines Local Car Care with "Transparency-First" Service Model in Santee
- Kilpack Panelist at 44th Annual Life, the Universe, and Everything Symposium
- SKIP, SKIP, HOORAY! Independent Publisher Crane Books Introduces Julia and the S.K.I.P. Movement—A Joyful Call to Show Kindness In Person
- Akron Authors Convention Announces Benjamin Wallace as 2026 Author Guest of Honor
- Indie Creator Opens the Digi 995 Universe Ahead of Kickstarter Launch
- Local Ohio Author Mark Bogner Releases Debut Novella The Dreamweaver
- Secondesk Launches Powerful AI Tutor That Speaks 20+ Languages
- Automation, innovation in healthcare processes featured at international conference in Atlanta
- A High-Velocity Growth Story Emerges in Marine and Luxury Markets
- $26 Billion Global Market by 2035 for Digital Assets Opens Major Potential for Currency Tech Company with ATM Expansion and Deployment Plans Underway
- Eye Spied: Serenity Acres The Prequel: Volume lll in the Thriller Series Now Available for Pre-Order
- Peernovation 365 is Now Available
- New Book by Juan Rodulfo Challenges the "Great Regression"