Popular on s4story
- Super League (N A S D A Q: SLE) Enters Breakout Phase: New Partnerships, Zero Debt & $20 Million Growth Capital Position Company for 2026 Acceleration - 168
- Russellville Huntington Learning Center Expands Access to Literacy Support; Approved Provider Under Arkansas Department of Education - 155
- Christy Sports donates $56K in new gear to SOS Outreach to help kids hit the slopes - 153
- Writing a Memoir About His Hometown of Quincy Mass. Turned Into a Search for His Missing Father - 152
- Entering 2026 with Expanding Footprint, Strong Industry Tailwinds, and Anticipated Q3 Results: Off The Hook YS Inc. (N Y S E American: OTH) - 147
- New Book "Downsize With Dignity" Helps Missouri Families Navigate Senior Moves - 127
- Phinge CEO Ranked #1 Globally by Crunchbase for the Last Week, Will Be in Las Vegas Jan. 4-9, the Week of CES to Discuss Netverse & IPO Coming in 2026 - 115
- T-TECH Partners with Japan USA Precision Tools for 2026 US Market Development of the New T-TECH 5-Axis QUICK MILL™
- UK Financial Ltd Announces A Special Board Meeting Today At 4PM: Orders MCAT Lock on CATEX, Adopts ERC-3643 Standard, & Cancels $0.20 MCOIN for $1
- Private Keys Are a Single Point of Failure: Security Advisor Gideon Cohen Warns MPC Technology Is Now the Only Defense for Institutional Custody
Similar on s4story
- Lick Personal Oils Introduces the Ultimate Valentine's Day Gift Collection for Romantic, Thoughtful Gifting
- Walmart $WMT and COSTCO.COM $COST Distribution as SonicShieldX™ Platform Sets the Stage for Accelerated Growth in 2026: AXIL Brands (N Y S E: AXIL)
- AI-Driven Drug Development with Publication of New Bioinformatics Whitepaper for BullFrog AI: $BFRG Strengthens Its Position in AI Drug Development
- IQSTEL Enters 2026 from a Position of Strength Following Transformational Year Marked by N A S D A Q Uplisting, Record Revenue and First-Ever
- Java Holdings LLC Acquires +Peptide, Expanding Portfolio Across Coffee, Science, and Functional Nutrition
- OneSolution® Expands to Orlando with New Altamonte Springs Implant Center
- 2025: A Turning Point for Human Rights. CCHR Demands End to Coercive Psychiatry
- UK Financial Ltd Executes Compliance Tasks Ahead Of First-Ever ERC-3643 Exchange-Traded Token, SMCAT & Sets Date For Online Investor Governance Vote
- HBZBZL Unveils "Intelligent Ecosystem" Strategy: Integrating AI Analytics with Web3 Incubation
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
$300 Million in Milestones Plus Tiered Double-Digit Royalties to License & Distribute NRX-100 Drug: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
S For Story/10653530
$NRXP is Poised to Address the $3 Billion + Suicidal Depression Market in the US
MIAMI - s4story -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
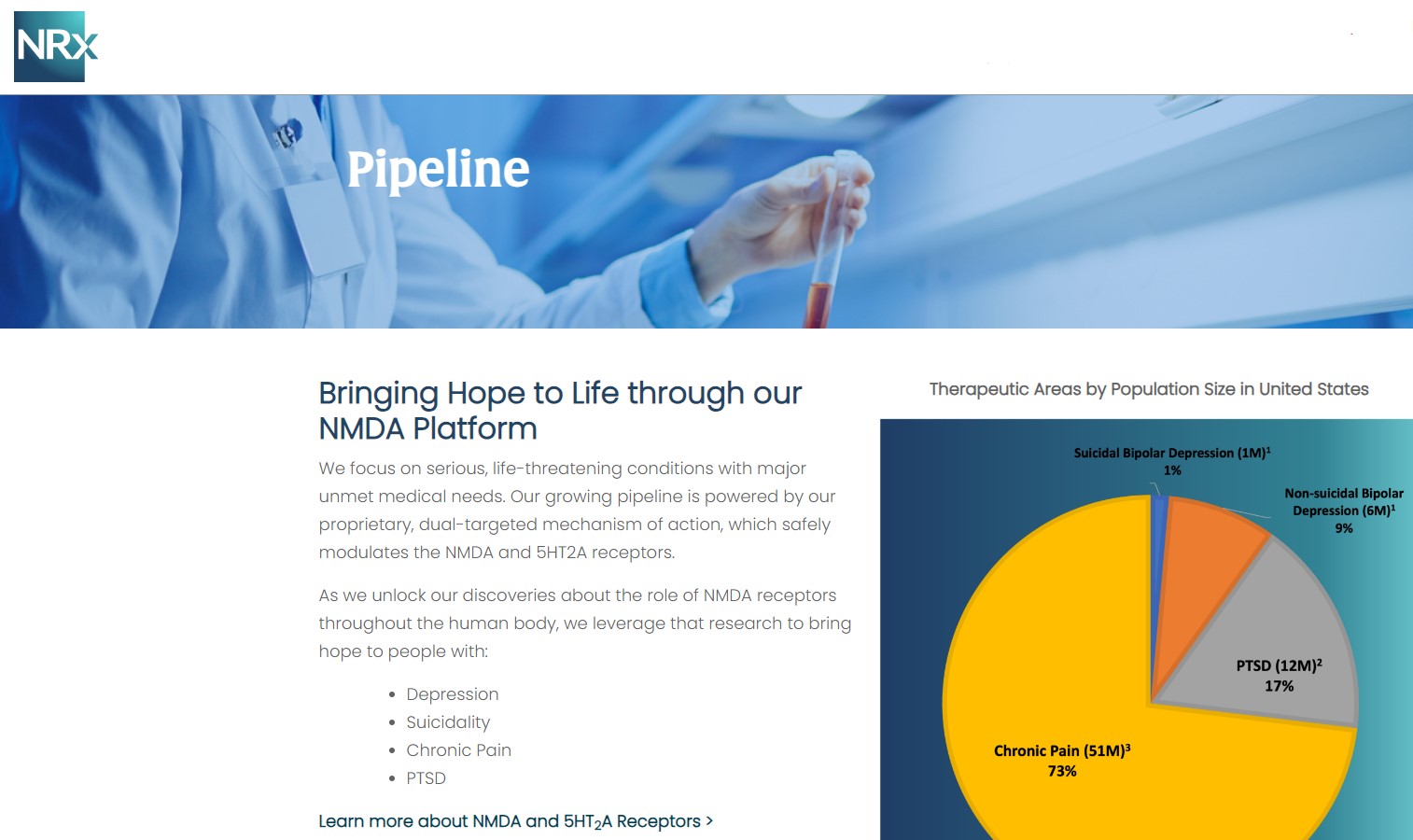
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
More on S For Story
Fourth Quarter and Full Year 2024 Financial Results and Provides Corporate Update
On March 17th NRXP announced its financial results for the quarter and year ended December 31, 2024, and provided a business update. The announcement included the following key highlights:
NRXP initiated filing of a New Drug Application ("NDA") to the FDA for NRX-100 (IV Ketamine) for the treatment of Suicidal Depression; planned filing of an NDA for Accelerated Approval under Breakthrough Designation and Priority Review of NRX-101 for the treatment of bipolar depression in people at risk of akathisia. Both have anticipated PDUFA dates prior to December 31, 2025
NRXP has accepted non-binding potential terms from a commercial pharmaceutical company to license and distribute NRX-100, providing over $300 million in milestones plus tiered double-digit royalties based on net sales
NRXP retained a leading regulatory law firm to file a citizen's petition with the US Food and Drug Administration ("FDA") to remove benzethonium chloride – a toxic preservative -- from presentations of ketamine intended for intravenous use; planned 2Q25 filing of an Abbreviated New Drug Application ("ANDA") for the use of preservative-free ketamine in all current indications
Wholly owned subsidiary HOPE Therapeutics, signed non-binding letters of intent to acquire three precision psychiatry centers and is currently completing financial due diligence and definitive agreements. Currently negotiating the terms for the acquisition of six additional centers
The HOPE acquisitions are planned to form the foundation for a national network offering interventional psychiatry services to treat suicidal depression, post-traumatic stress disorder ("PTSD") and related conditions
NRXP received and negotiating a term sheet from a publicly-traded strategic investor currently engaged in manufacturing Transcranial Magnetic Stimulation ("TMS") devices to provide capital in support of expansion of further HOPE clinic acquisitions.
NRXP has engaged BTIG as financial advisor for clinic acquisition and capital formation; leading global financial services firm specializing in investment banking, institutional trading, research, and related brokerage services for strategic growth opportunities.
NRXP regained compliance with the NASDAQ market value of listed securities ("MVLS") requirement.
Substantially reduced operating costs compared to prior year
Management continues to forecast, although no assurances can be given, profitability on a forward-looking run-rate basis by year end 2025
More on S For Story
NRXP filed Module 3 (manufacturing) of its New Drug Application ("NDA") for NRX-100 (preservative-free sterile IV ketamine) in a tamper-resistant, diversion resistant packaging presentation in the fourth quarter of 2024. NRX-100 was previously granted Fast Track Designation by FDA in combination with use of NRX-101. Ketamine efficacy data from four clinical trials are intended to support the filing. Three manufacturing lots are now complete, with filed stability data suitable for shelf life exceeding two years at room temperature. The anticipated PDUFA date for this NDA is prior to December 31, 2025.
NRX-100 is poised to address the over $3 billion Suicidal Depression market in the US.
NRXP has accepted non-binding potential terms from a commercial pharmaceutical company to license and distribute NRX-100 (preservative-free IV ketamine) that provides for over $300 million in potential milestones plus a tiered double-digit royalty, subject to further due diligence and finalized agreement.
NRXP announced its intention to file an NDA for Accelerated Approval under Breakthrough Designation and Priority Review of NRX-101 for the treatment of bipolar depression in people at risk of akathisia, based on the Phase 2b/3 and STABIL-B data. Three manufacturing lots are now complete with more than 12 months of room temperature shelf-stability. The anticipated PDUFA date for this application is prior to December 31, 2025.
NRXP estimates that the market for the initial indication is over $2 billion, while the broad bipolar market could exceed $5 billion.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Application to Uplist to NASDAQ Global Market from NASDAQ Capital Market
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
More on S For Story
- Walmart $WMT and COSTCO.COM $COST Distribution as SonicShieldX™ Platform Sets the Stage for Accelerated Growth in 2026: AXIL Brands (N Y S E: AXIL)
- AI-Driven Drug Development with Publication of New Bioinformatics Whitepaper for BullFrog AI: $BFRG Strengthens Its Position in AI Drug Development
- IQSTEL Enters 2026 from a Position of Strength Following Transformational Year Marked by N A S D A Q Uplisting, Record Revenue and First-Ever
- Are You Hiring The Right Heater Repair Company in Philly?
- Appliance EMT Expands Professional Appliance Repair Services to Hartford, Connecticut
Fourth Quarter and Full Year 2024 Financial Results and Provides Corporate Update
On March 17th NRXP announced its financial results for the quarter and year ended December 31, 2024, and provided a business update. The announcement included the following key highlights:
NRXP initiated filing of a New Drug Application ("NDA") to the FDA for NRX-100 (IV Ketamine) for the treatment of Suicidal Depression; planned filing of an NDA for Accelerated Approval under Breakthrough Designation and Priority Review of NRX-101 for the treatment of bipolar depression in people at risk of akathisia. Both have anticipated PDUFA dates prior to December 31, 2025
NRXP has accepted non-binding potential terms from a commercial pharmaceutical company to license and distribute NRX-100, providing over $300 million in milestones plus tiered double-digit royalties based on net sales
NRXP retained a leading regulatory law firm to file a citizen's petition with the US Food and Drug Administration ("FDA") to remove benzethonium chloride – a toxic preservative -- from presentations of ketamine intended for intravenous use; planned 2Q25 filing of an Abbreviated New Drug Application ("ANDA") for the use of preservative-free ketamine in all current indications
Wholly owned subsidiary HOPE Therapeutics, signed non-binding letters of intent to acquire three precision psychiatry centers and is currently completing financial due diligence and definitive agreements. Currently negotiating the terms for the acquisition of six additional centers
The HOPE acquisitions are planned to form the foundation for a national network offering interventional psychiatry services to treat suicidal depression, post-traumatic stress disorder ("PTSD") and related conditions
NRXP received and negotiating a term sheet from a publicly-traded strategic investor currently engaged in manufacturing Transcranial Magnetic Stimulation ("TMS") devices to provide capital in support of expansion of further HOPE clinic acquisitions.
NRXP has engaged BTIG as financial advisor for clinic acquisition and capital formation; leading global financial services firm specializing in investment banking, institutional trading, research, and related brokerage services for strategic growth opportunities.
NRXP regained compliance with the NASDAQ market value of listed securities ("MVLS") requirement.
Substantially reduced operating costs compared to prior year
Management continues to forecast, although no assurances can be given, profitability on a forward-looking run-rate basis by year end 2025
More on S For Story
- Java Holdings LLC Acquires +Peptide, Expanding Portfolio Across Coffee, Science, and Functional Nutrition
- First Audiobook Wins Outstanding Creator Awards For Historical Fiction & Other Categories
- Author Michelle Carey Releases Free Fiction and Non-Fiction eBooks to Spark Creative Growth
- OneSolution® Expands to Orlando with New Altamonte Springs Implant Center
- Beyond the Book: Clarity & Purpose with Award-Winning Author Renee Daniel Flagler
NRXP filed Module 3 (manufacturing) of its New Drug Application ("NDA") for NRX-100 (preservative-free sterile IV ketamine) in a tamper-resistant, diversion resistant packaging presentation in the fourth quarter of 2024. NRX-100 was previously granted Fast Track Designation by FDA in combination with use of NRX-101. Ketamine efficacy data from four clinical trials are intended to support the filing. Three manufacturing lots are now complete, with filed stability data suitable for shelf life exceeding two years at room temperature. The anticipated PDUFA date for this NDA is prior to December 31, 2025.
NRX-100 is poised to address the over $3 billion Suicidal Depression market in the US.
NRXP has accepted non-binding potential terms from a commercial pharmaceutical company to license and distribute NRX-100 (preservative-free IV ketamine) that provides for over $300 million in potential milestones plus a tiered double-digit royalty, subject to further due diligence and finalized agreement.
NRXP announced its intention to file an NDA for Accelerated Approval under Breakthrough Designation and Priority Review of NRX-101 for the treatment of bipolar depression in people at risk of akathisia, based on the Phase 2b/3 and STABIL-B data. Three manufacturing lots are now complete with more than 12 months of room temperature shelf-stability. The anticipated PDUFA date for this application is prior to December 31, 2025.
NRXP estimates that the market for the initial indication is over $2 billion, while the broad bipolar market could exceed $5 billion.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on S For Story
- HBZBZL Unveils "Intelligent Ecosystem" Strategy: Integrating AI Analytics with Web3 Incubation
- Kaltra Launches Next-Gen MCHEdesign With Full Integration Into MCHEselect — Instant Simulation & Seamless Microchannel Coil Workflow
- A Well-Fed World, Youth Climate Save and PAN International Launch PHRESH: A Global Directory of Plant-Based Hunger Relief Organizations
- 4-Hour Work Day: Jon Robert Quinn Challenges Hustle Culture and Redefines Entrepreneurial Success
- New Book "Downsize With Dignity" Helps Missouri Families Navigate Senior Moves
- Guests Can Save 25 Percent Off Last Minute Bookings at KeysCaribbean's Village at Hawks Cay Villas
- Trump's Executive Order Rescheduling Cannabis: Accelerating M&A in a Multibillion-Dollar Industry
- "Wayfarers" a Short Story by Algernon Blackwood Now Available on Audiobook
- Genuine Hospitality, LLC Selected to Operate Hilton Garden Inn Birmingham SE / Liberty Park
- Kilpack's Sci-Fi Novella Pale Face Named Finalist in Literary Global Book Awards
- Documentary "Prescription for Violence: Psychiatry's Deadly Side Effects" Premieres, Exposes Link Between Psychiatric Drugs and Acts of Mass Violence
- Price Improvement on Luxurious Lāna'i Townhome with Stunning Ocean Views
- Nextvisit Co-Founder Ryan Yannelli Identifies Six Critical Factors for Behavioral Health Providers Evaluating AI Scribes in 2026
- CredHub and Real Property Management Join Forces to Empower Franchise Owners with Rental Payment Credit Reporting Solutions
- Dodd, Mead & Company Revived Under Current Trademark Ownership
- Leimert Park Announces Weeklong Kwanzaa Festival & Kwanzaa Parade Celebrating Black History, Culture, and Community
- Independent Author Launches National Registry Following $16M Federal Antitrust Complaint
- Renowned Alternative Medicine Specialist Dr. Sebi and His African Bio Mineral Balance Therapy Are the Focus of New Book
- Psychiatric Drug Damage Ignored for Decades; CCHR Demands Federal Action
- Why Millions Are Losing Sexual Sensation, And Why It's Not Age, Hormones, or Desire