Popular on s4story
- Keepy Uppy™ by Ollyball Wins Prestigious 2025 Influencer Award from Clamour & The Toy Association; Announces Fall 2025 Launch at Target Stores
- Venardi Zurada LLP Offers Legal Support to Families After Deadly Lake Tahoe Boat Capsizing
- Jasmine Farrell Releases New LGBTQ+ Poetry Collection - Rising From the Roots
- K2 Integrity's U.S. and EMEA Teams Recognized in Chambers and Partners 2025 Guides
- Elevated Healing Treatment Centers: Redefining Mental Health Care with Compassionate, Evidence-Based, and Accessible Services
- Holiday Inn Express North Hollywood Burbank Area Announces Conversion to Hampton Inn North Hollywood
- ASI Accelerates iMIS® Innovation by Acquiring CSI's Product Suite and Expert Team
- Lottery.com Inc. Secures $300 Million in Growth Capital, Confirms Nasdaq Compliance & Acquires UAE Sports Incubator Amid High-Profile Brand Exposure
- Anna D. Banks' Street Smart, Money Smart Hits #1 on Amazon Teen & Young Adult New Releases Chart
- Nationally Recognized Hispanic Activist and Businessman, Luis Figueroa, to speak at CPAC Latino 2025, Showcasing Hispanic Leadership in Action
Similar on s4story
- The Blue Luna Encourages Local Schools to Take Steps to Enhance Safety for Students and Staff
- Smart Resnse Unveils Smart Resnse(SRMS) Token-Powered AI Orchestration Platform to Revolutionize Multi-Billion Dollar Market
- Josh and Heidi Follow Up the Much Anticipated and Successful Launch of the "Spreading the Good BUZZ" Podcast with a Personal Request
- Revolutionary Blockchain Platform Okh Finance Announces Okh Finance(OKKH) Token Launch to Transform Global Asset Leasing Market
- Stuck Doing Math or Figuring Out Life's Numbers? Calculator.now Makes It Stupidly Simple
- The World's Largest Green Economic Revolution Emerges as Nature, Tech, and Finance Converge
- Vinnetwork Unveils Decentralized AI Platform with Vinnetwork(VIN) Token to Challenge Tech Giants' Data Monopoly
- Pyro Marketing Opens New Digital Marketing Company to Power Growth for Fitness and Ecommerce Brands
- Dr. John Salerno of Salerno Wellness Introduces Their New Full Body Capsule for Advanced LED Light Therapy Patient Treatments
- $14M Expansion Deal with Famed David Lloyd Highlights Rebrand of Sports, Entertainment and Gaming Innovation by AI Driven, Online Fan Engagement Co
CEO Selected to Present at Wall Street Conference on May 21, 2025 for Suicidal Depression / PTSD: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
S For Story/10660124
$NRXP Closing $7.8 Million in Financing for Clinic Acquisitions and Purchase of Kadima Neuropsychiatry Institute Treatment Model and Leading Investigative Site
MIAMI - s4story -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to the Global Market from Capital Market
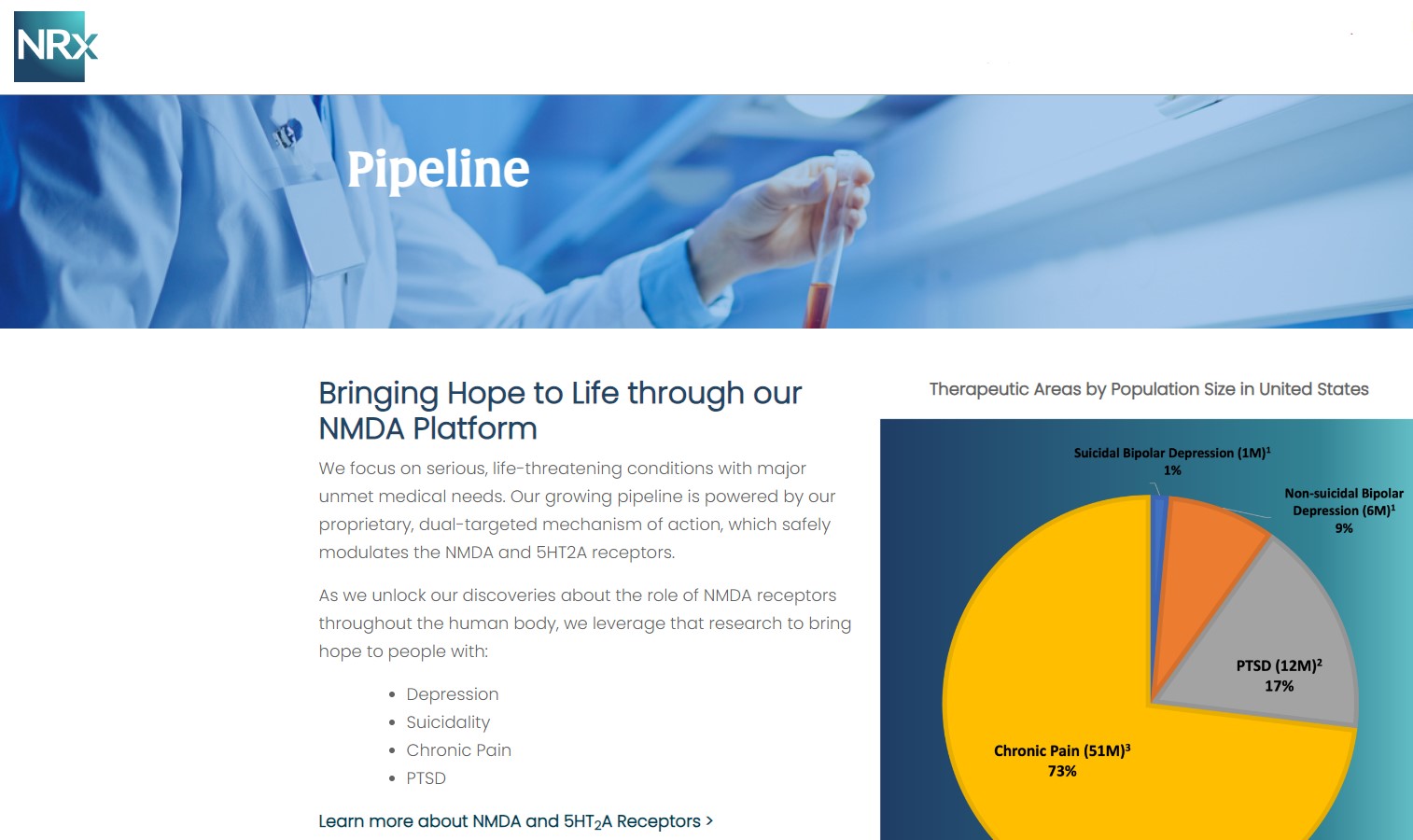
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
More on S For Story
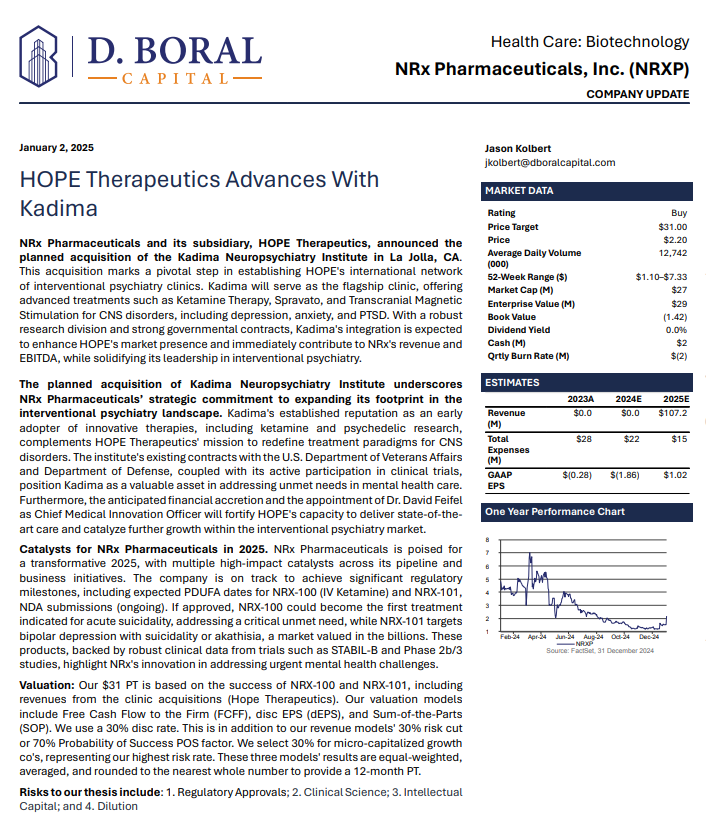
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
NRx Pharmaceuticals Selected to Present at the Wall Street Conference on May 21, 2025, in Palm Beach, Florida
On May 21st NRXP announced that that Jonathan Javitt, MD, MPH, Founder, Chairman and Chief Executive Officer of NRx Pharmaceuticals and Co-CEO of HOPE Therapeutics, will be presenting a Company update at the Wall Street Conference, taking place the same day in Palm Beach, FL. NRXP is one of six companies invited to present.
The Wall Street Conference is expected to host over 1,000 attendees who are reported to represent over $1T in investment capital. Wall Street Conference Home
NRXP will be discussing recent progress towards FDA approval of NRX-100 (preservative-free ketamine) and upcoming acquisitions of HOPE clinics providing state-of-the-art care for suicidal depression, PTSD, and related disorders.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
More on S For Story
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, transcranial magnetic stimulation ("TMS") as well as medication management. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact:
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Aiming to be the First FDA-Approved Medication to Treat Suicidal Depression
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Added to Previously Announced Term Sheet with a Strategic Investor, NRXP HOPE Funding of $10.3 Million is Planned in Near Term.
Agreement to Purchase Kadima Neuropsychiatry Institute Expected to Serve as Clinical Model for Treatment Offerings Nationwide.
Kadima is a Leading Investigative Site for CNS and Psychedelic Research, Having Served as the Lead Site in Nearly All Major Trials in This Space.
Dr. David Feifel, Nationally Recognized Pioneer in Interventional Psychiatry to join as Chief Medical Innovation Officer.
New Drug Application for Treatment of Suicidal Depression; Planned NDA for Accelerated Approval for Bipolar Depression in People at Risk of Akathisia.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine. Patent expected to be Orange Book Listable.
Waiver Exemption from Paying a $4.3 Million New Drug Application Fee Under Prescription Drug User Fee Act (PDUFA).
Company On Track for Q2 2025 Completion of NDA Filing and PDUFA Date by Year End with Currently Available Resources.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
Poised to Address Over $3 Billion Suicidal Depression Market in the US.
Application to Uplist to the Global Market from Capital Market
NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year. This represents a $3-5 billion market at expected pricing. Based on the data in the trials referenced above, the Company's regulatory counsel encouraged the Company to file an NDA for suicidal depression for NRX-100.
More on S For Story
- Emmy-Winning Journalist José Martínez Releases Debut Book Your English is Great, But…
- The Blue Luna Encourages Local Schools to Take Steps to Enhance Safety for Students and Staff
- The Sessions Studios Secures $300 Million Commitment to Launch World-Class Studio and 15-Film Global Slate
- Smart Resnse Unveils Smart Resnse(SRMS) Token-Powered AI Orchestration Platform to Revolutionize Multi-Billion Dollar Market
- Josh and Heidi Follow Up the Much Anticipated and Successful Launch of the "Spreading the Good BUZZ" Podcast with a Personal Request
In a January 2025 report, respected investment analysis firm D. Boral Capital assigned NRXP a $31 Price Target. The full report may be viewed at this link: https://www.nrxpharma.com/wp-content/uploads/2025/01/HOPE-Therapeutics-Advances-With-Kadima.pdf.
NRx Pharmaceuticals Selected to Present at the Wall Street Conference on May 21, 2025, in Palm Beach, Florida
On May 21st NRXP announced that that Jonathan Javitt, MD, MPH, Founder, Chairman and Chief Executive Officer of NRx Pharmaceuticals and Co-CEO of HOPE Therapeutics, will be presenting a Company update at the Wall Street Conference, taking place the same day in Palm Beach, FL. NRXP is one of six companies invited to present.
The Wall Street Conference is expected to host over 1,000 attendees who are reported to represent over $1T in investment capital. Wall Street Conference Home
NRXP will be discussing recent progress towards FDA approval of NRX-100 (preservative-free ketamine) and upcoming acquisitions of HOPE clinics providing state-of-the-art care for suicidal depression, PTSD, and related disorders.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions
On May 15th NRXP announced signing of a term sheet with Universal Capital, LLC to provide $7.8 million in acquisition capital to initiate subsidiary HOPE's planned national rollup of interventional psychiatry clinics, commencing with previously-announced acquisitions of Dura Medical, Kadima, and NeuroSpa. Together with proceeds of a previously announced strategic investment, this financing is anticipated to provide $10.3 million in acquisition capital.
HOPE's three initial acquisitions represent best-in-class clinics that offer neuroplastic treatments including NRXP ketamine and transcranial magnetic stimulation (TMS) to treat patients with severe depression, PTSD, and related central nervous system conditions. Neuroplastic treatments represent a rapidly emerging class of interventions that cause the growth of new connections (synapses) between brain cells that have been shown in multiple clinical trials to relieve symptoms of depression and suicidality. The FDA has approved TMS devices for a number of indications and has approved a nasal form of ketamine for treatment resistant depression. HOPE's parent company, NRXP, is currently applying to the FDA for approval of intravenous preservative-free ketamine to treat suicidal depression.
Agreement to Purchase Kadima Neuropsychiatry Institute; Foundational Acquisition for the NRXP HOPE Network of Interventional Psychiatry Clinics
On May 13th NRXP announced signing of a definitive agreement to purchase the Kadima Neuropsychiatry Institute. Kadima is expected to serve as the clinical model for treatment offerings in NRXP HOPE-acquired clinics and is expected to continue its role as a leading investigative site for research into neuroplastic therapies including psychedelic medications, transcranial magnetic stimulation (TMS), and hyperbaric therapy.
More on S For Story
- Revolutionary Blockchain Platform Okh Finance Announces Okh Finance(OKKH) Token Launch to Transform Global Asset Leasing Market
- Cover Girl Finalist Teisha Mechetti Questions Legitimacy of Inked Originals Competition, Demands Transparency
- Author Launches The Starlight Bond Website with Movie Licensing Proposal
- Easton & Easton, LLP Files Suit Against The Dwelling Place Anaheim & Vineyard USA Over Abuse Allegations
- AI Visibility: The Key to Beating Google's AI Overviews and Regaining Traffic
Kadima is one of California's flagship interventional psychiatry clinics and was among the first to bring ketamine treatment for central nervous system (CNS) disorders out of academic research settings and into clinical practice. The clinic offers a full range of treatments for suicidal depression, PTSD and other CNS disorders, including ketamine, Spravato, transcranial magnetic stimulation ("TMS") as well as medication management. Further, Kadima and David Feifel, MD PhD, Founder and Medical Director of Kadima, have served as leaders in clinical trial work on emerging therapies in CNS for top companies in the industry. Importantly, the clinic is profitable and is forecast to continue growth going forward. Dr. Feifel will join NRXP HOPE as its first Chief Medical Innovation Officer upon closing of the acquisition.
Patent Application Filed for NRX-100 Proprietary, Preservative Free Formulation of IV Ketamine
On May 5th NRXP announced the filing of a patent application for NRX-100, its preservative-free intravenous ketamine formulation for the treatment of suicidal depression. The application discloses pharmaceutical compositions, methods of treatment and methods of manufacture and currently includes twenty claims. While subject to the patent review process of the US Patent and Trademark Office, if granted, the patent would provide NRX-100 exclusivity into 2045.
This patent filing builds on the NRXP recently initiated filing of an NDA for NRX-100 and its prior Fast Track Designation, with NRX-101, from the FDA. If granted, the patent will help protect the innovation behind this formulation as NRXP advances its commercialization strategy.
FDA Award of Filing Fee Waiver for Upcoming NRX-100 (preservative free ketamine) New Drug Application to Treat Patients with Suicidal Depression
On April 30th NRXP announced the grant of a filing fee waiver by the US Food and Drug Administration ("FDA") to exempt the Company from a $4.3 million fee to file its New Drug Application for NRX-100 (preservative-free ketamine). The waiver is granted at the discretion of the FDA to Small Business Entities and for drugs that are deemed to be necessary for Public Health. NRXP anticipates that this waiver enables the completion of its New Drug Application for NRX-100 with currently-available corporate resources. The NDA filing is anticipated by the end of the second quarter of this year (Q2 2025).
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact:
Company Name: NRx Pharmaceuticals, Inc. (Stock Symbol: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Health
0 Comments
Latest on S For Story
- Former Teacher to Dr. Phil's Critique: "Unschooling Isn't Chaos — It's the Future"
- Heartfelt Dreams Foundation Launches Campaign to Build CHD Hospital
- Radarsign Tackles Intersection Safety with Launch of Grid-Free Solar LED Stop Sign
- Curtis Sergeant's Book The Only One Continues to Equip Christians to Live Fully in by and for God
- Emmy-Winning Journalist José Martínez to Debut Powerful New Book at New York Mobile Film Festival
- Miami Real Estate Agent Drastically Increases Interest In Homes
- Adostics & Genmega Announce the Introduction of A-POD
- LIB and Nidec Rejoin Forces for Giant TH-0098 Temperature Humidity Test Chamber
- Wordeee Publishes Casting Pros to Know: Reality TV Edition by Asjai Lou
- Digi 995 Audiobooks Officially Released: Fans Can Now Listen to the Complete Trilogy
- Heritage at South Brunswick Offers Immediate Townhome Appointments and Special Mortgage Incentive Fast-Moving Sales
- New Children's Book Helps Kids and Parents Navigate Anxiety Together
- New TSA-Compliant Medication Packing Tool Helps Travelers Avoid Airport Delays and Customs Issues
- Wordeee Publishes Am I a Weed? by Margie Stiles
- NASA Collaborative Agreement for Supply of Thin-Film Solar Tech for Orbital Application to Advance Development of Thin-Film PV Power Beaming: $ASTI
- Sci-Fi Novel from Pittsburgh Author Explores Love, Power, & Humanity in an Age of Artificial People
- Exciting New Era of Sports, Entertainment & Gaming Innovation Spotlighted by Rebrand of Expanding AI Driven, Online Fan Engagement Company: SEGG Media
- Service Ninjas Debuts First-of-Its-Kind "Membership" Platform for Home Service Pros
- The Journey of BECOMING the Soul Alchemist — New Book by Kay Sanders Guides Readers to Deep Inner Transformation
- BIYA Forecasts 2025 Surge with ¥300M ($41.8 M USD) in Revenue and ¥25M Profit from Cloud Based HR Solutions: Baiya Intl. Group (N A S D A Q: BIYA)