Popular on s4story
- Libraries for Kids International Announces 2026 Board of Directors - 152
- Tawanna Chamberlain Launches New Book, Outsized Ambition: The Blueprint for Going Beyond! - 117
- Phillip E Walker's EntryLevelActing.com Actor Employment Advice E-Book Road Map Launches on MLK Day
- For Valentine's Day: Treat yourself (and maybe even your sweetheart) to some Not Exactly Love Poems
- New Anthology Release by Dark Moon Books: HORROR LIBRARY, VOLUME 9
- New Middle Grade Novel A New Way to Know Releases February 2, 2026
- 2026 Grateful American Book Prize Call for Submissions
- Hunter AKA Mark E. Hunter Is Off and Running with 2026 New Music and Tour
- Q3 2025 Arizona Technology Industry Impact Report Highlights Shifting Job Demand, Semiconductor Momentum and Workforce Investment
- Author Gate Strengthens Traditional Publishing Through Investment in Writers and Editors
Similar on s4story
- Parkway Prosthodontics Achieves Breakthrough Full-Arch Reconstruction Case
- Postmortem Pathology Expands to Phoenix: Bringing Families Answers During Their Most Difficult Moments
- Blasting Off with Space Sector Companies: Artemis II Manned Moon Mission is Set to Launch: Could $ASTI be on the Same Rocket Ride as $ASTS & $LUNR?
- HBMHCW Expande Infraestructura de Cumplimiento para Argentina mientras América Latina Supera $1.5 Billones en Volumen Cripto
- Norisia Launches AI Formulated Luxury Multivitamin to Transform Daily Wellness in the UK
- NASA / Glenn Research Center Collaboration to Help Meet Rising Demand for Space Energy Beaming Tech / CIGS PV Modules from Ascent Solar: NAS DAQ: ASTI
- When Interpretation Becomes Conversation: Rethinking Engagement in the Museum Age
- RTC Communications Completes Next Level Connect Fiber Expansion Bringing Multi-Gig Broadband to West Boggs Community
- EPP Pricing Platform announces leadership transition to support long-term growth and continuity
- Roshni Online Services Unveils Plans for Innovative Digital Consultation Platform
Lineus Medical Completes UK Registration for SafeBreak® Vascular
S For Story/10681404
FAYETTEVILLE, Ark. - s4story -- Lineus Medical is now officially registered in the United Kingdom, enabling the company to begin distributing SafeBreak® Vascular within the UK healthcare market. UK registration represents an important step in Lineus Medical's international expansion strategy, further extending access to SafeBreak Vascular for hospitals and clinicians on a global scale. With regulatory requirements in place, Lineus Medical can now work with distribution partners to bring its breakaway IV technology to the UK.
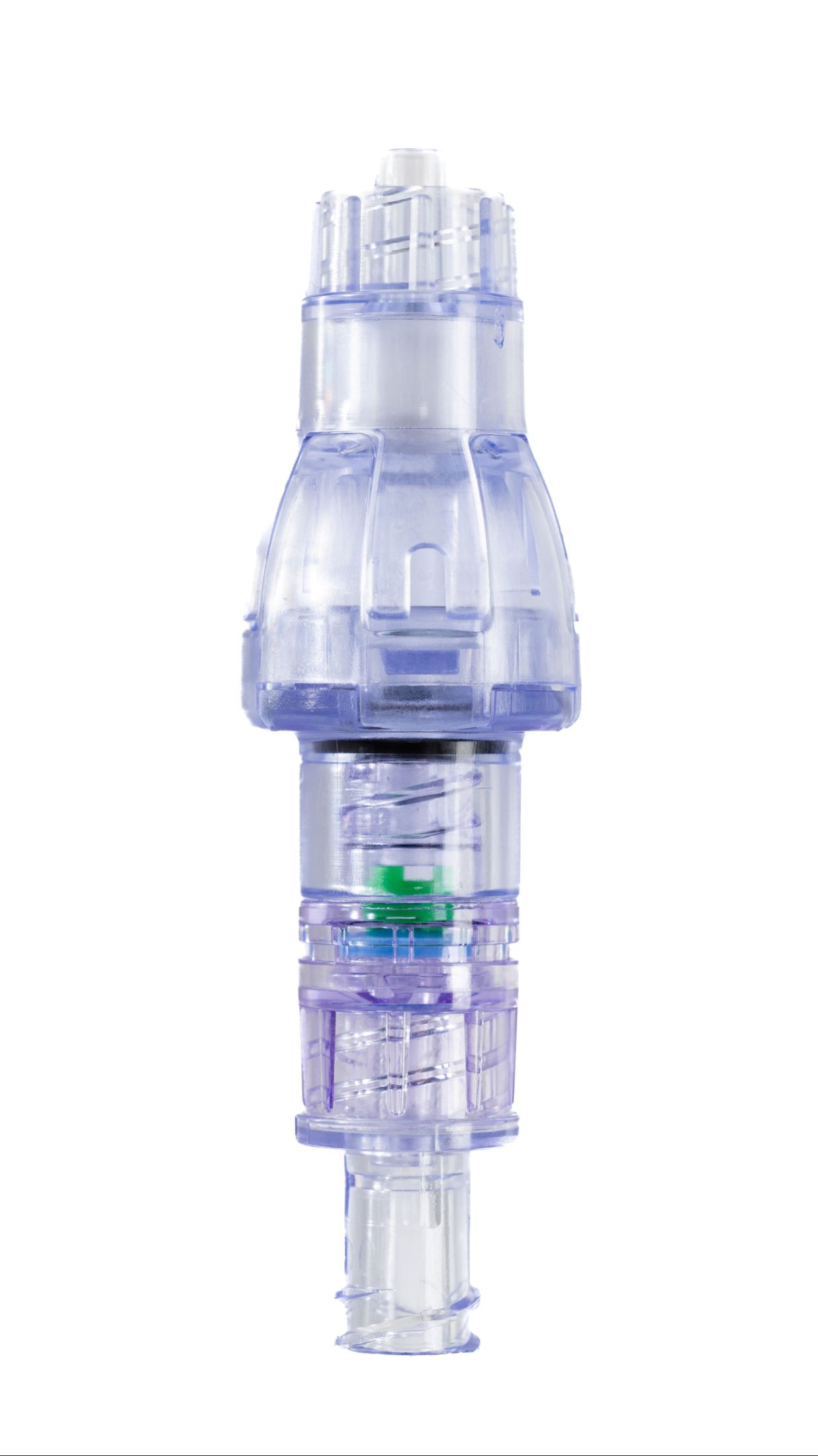
SafeBreak Vascular is a breakaway device for IV lines clinically proven to reduce IV complications.¹ When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When separation occurs, valves on both ends of the device close to prevent medication spills from the pump and blood loss from the patient. Patients avoid additional needlesticks, nurses save time, and hospitals save money.¹
More on S For Story
"Completing UK registration is another meaningful milestone as we continue to expand access to SafeBreak globally," said Vance Clement, CEO of Lineus Medical. "Each new market brings us closer to our mission of removing the pains associated with IV lines and improving patient safety across healthcare systems worldwide. UK registration allows SafeBreak to reach another 69 million people."
"This registration allows us to move forward with distribution planning in the UK and supports our broader international commercialization efforts," said Larry Hayes, Chief Commercial Officer of Lineus Medical. "We are focused on working with the right partners to ensure SafeBreak Vascular is accessible to clinicians who are looking to reduce IV complications and improve care at the bedside."
About Lineus Medical:
Lineus Medical is the developer of SafeBreak® Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References
SafeBreak Vascular is a breakaway device for IV lines clinically proven to reduce IV complications.¹ When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When separation occurs, valves on both ends of the device close to prevent medication spills from the pump and blood loss from the patient. Patients avoid additional needlesticks, nurses save time, and hospitals save money.¹
More on S For Story
- Blasting Off with Space Sector Companies: Artemis II Manned Moon Mission is Set to Launch: Could $ASTI be on the Same Rocket Ride as $ASTS & $LUNR?
- redrosethorns Announces Second Book Acquisition of a Romantasy Novelette by Christin Marie
- Costa Oil Named Primary Sponsor of Carson Ware for the United Rentals 300 at Daytona International Speedway
- HBMHCW Expande Infraestructura de Cumplimiento para Argentina mientras América Latina Supera $1.5 Billones en Volumen Cripto
- Norisia Launches AI Formulated Luxury Multivitamin to Transform Daily Wellness in the UK
"Completing UK registration is another meaningful milestone as we continue to expand access to SafeBreak globally," said Vance Clement, CEO of Lineus Medical. "Each new market brings us closer to our mission of removing the pains associated with IV lines and improving patient safety across healthcare systems worldwide. UK registration allows SafeBreak to reach another 69 million people."
"This registration allows us to move forward with distribution planning in the UK and supports our broader international commercialization efforts," said Larry Hayes, Chief Commercial Officer of Lineus Medical. "We are focused on working with the right partners to ensure SafeBreak Vascular is accessible to clinicians who are looking to reduce IV complications and improve care at the bedside."
About Lineus Medical:
Lineus Medical is the developer of SafeBreak® Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References
- Data on file.
Source: Lineus Medical
0 Comments
Latest on S For Story
- History Matters: Book Recommendations for February
- Half of Finnish Online Gambling Expenditure Now Flows to Offshore Instant Casinos as License Applications Open March 1, 2026
- RTC Communications Completes Next Level Connect Fiber Expansion Bringing Multi-Gig Broadband to West Boggs Community
- EPP Pricing Platform announces leadership transition to support long-term growth and continuity
- Stolen Hearts: Reclaiming Your Child From Parental Alienation (narcissistic abuse)
- Roshni Online Services Unveils Plans for Innovative Digital Consultation Platform
- The Wolf Experiment by Laura Daleo
- Barringer Publishing Announces the Publication of "Three Black Swans"
- Stuart Smith Releases New Zombie Survival Novel - The Last Sanctuary of the Living
- Wall Street Is Missing This One: Cycurion (NAS DAQ: CYCU) Gets $7 Price Target While Trading at a Steep Discount
- Aries Industries Streamlines Sewer Inspection Process With Introduction of the LETS Sidewinder
- Publishing Expert Dawn James Launches Your Memoir Blueprint to Help Families Preserve Their Stories
- Chronic Boss Awards Scholarships to Student Founders Living with Chronic Conditions
- Nest Finders Property Management Named #1 in Jacksonville and Ranked #99 Nationwide
- New Release by Lizzy Stevens And Steve Miller
- Nashville International Chopin Piano Competition Launches First Amateur Edition
- A Boulder Programmer Lost his Wife in 2022 – This Year, They Wrote a Book Together
- Market Value Enhancement From 2 Important New US Patents Issued for Strengthening Hair Enzyme Booster Technology to Caring Brands (NAS DAQ: CABR)
- HELM Audio™ Partners with PQCrypto to Future-Proof Children's Hearing and Safety Data Using Post-Quantum Cryptography
- Wala Blegay to Announce Run for Congress in Maryland's 5th District on Feb. 4