Popular on s4story
- The Office of Count Jonathan of Aquitaine Establishes Centre for Education and Diplomacy - 464
- The 2025 "Aizu Festival" in Aizu Wakamatsu City will be held September 19–21 - 440
- Bookmakers Review: Joe Rogan Favored to Win Inaugural 2025 Golden Globes Podcast of the Year - 433
- Ubleu Crypto Group Achieves FinCEN Registration and Colorado Incorporation, Accelerating U.S. Market Entry - 430
- New Urban Fantasy Series 'Secret Empires' Brings Ancient Magic and Hidden Wars to Life - 429
- Iterators Named Preferred Accessibility Testing Vendor by MIT - 423
- Memoir Surge and Publishing Innovation: Independent Houses Lead the Next Chapter of Literary Culture - 366
- Love Death + Explosives: Thomas Pynchon's Polipsychology | An Essay by Michael Finney - 353
- Sober.Buzz Adds Second Podcast, "Spreading the Good BUZZ" Guest List Grows, Numbers Continue Growing Globally, All While Josh and Heidi Tied the Knot - 340
- Cuesta College Central Coast Writers' Conference Announces Scholarship Contests, Teen Program, and Vendor Opportunities - 216
Similar on s4story
- CCHR: For Prevention, Families Deserve Truth From NIH Study on Psychiatric Drugs
- Sheets.Market Brings Professional Financial Model Templates to Entrepreneurs and Startups
- Webinar Announcement: Investing in the European Defense Sector—How the New Era of Uncertainty Is Redefining Investment Strategies
- AEVIGRA (AEIA) Analysis Reveals $350 Billion Counterfeit Market Driving Luxury Sector Toward Blockchain Authentication
- $40 Price Target for $NRXP in H. C. Wainright Analyst Report on Leader in $3 Billion Suicidal Depression Market with Superior NRX 100 Drug Therapy
- AHRFD Releases Market Analysis: Cryptocurrency Market's Institutional Transformation Accelerating
- Ubleu Crypto Group Analyzes European Digital Asset Market Opportunities Amid Regulatory Evolution
- NIUFO Examines European MiCA Regulation's Impact on Digital Asset Trading Markets
- Wzzph Analyzes Crypto Market Maturation as Institutional Capital Drives $50B ETF Inflows
- GXCYPX Analyzes South America's Emerging Digital Asset Market Dynamics
$3 Billion Suicidal Depression Market Advancements on Multiple Fronts, Highlighted by FDA Fast Track Designation for Effective NRX 100 Drug Therapy
S For Story/10668997
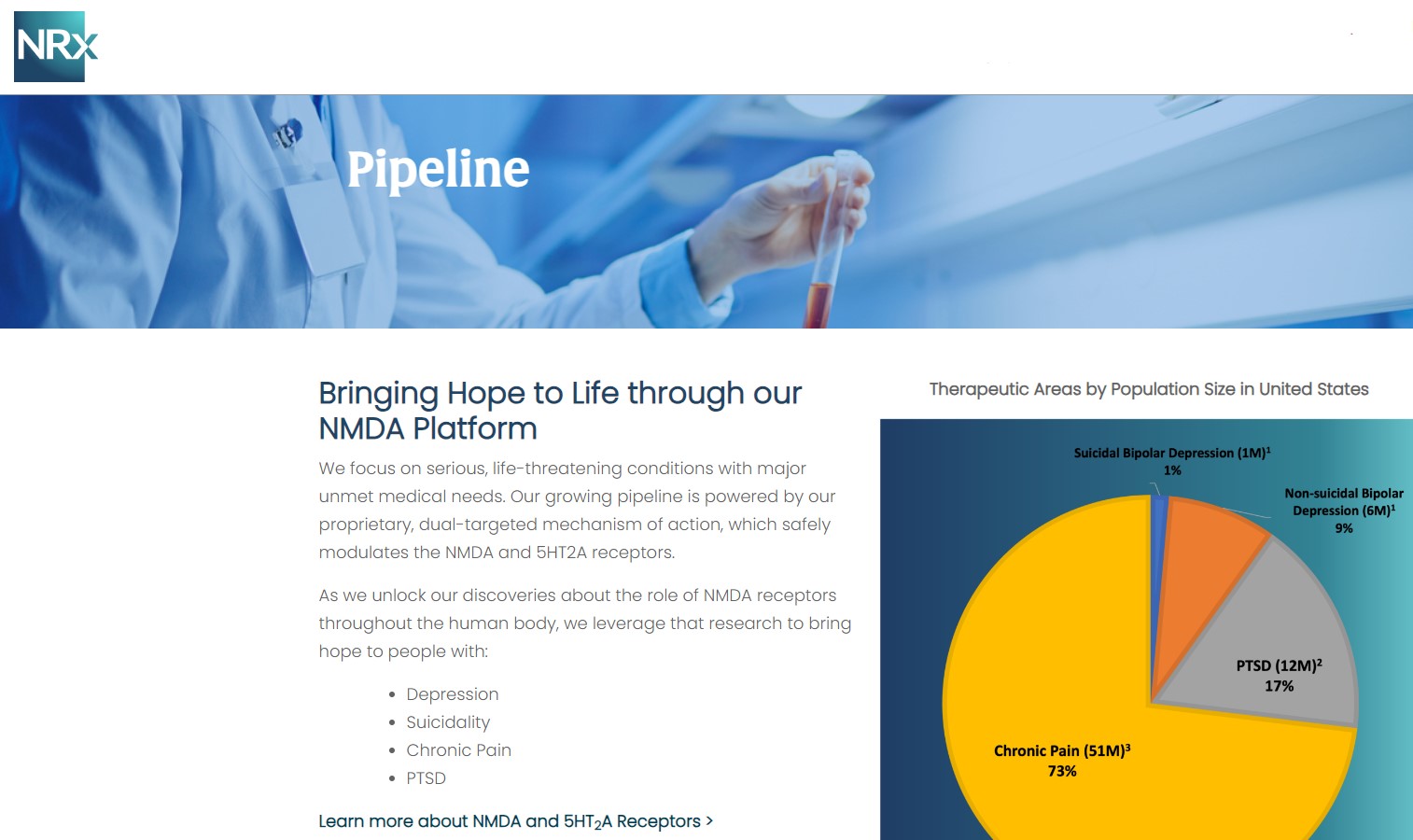
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP Sees 10-Fold Market Expansion to 13 Million Americans for Bipolar Depression Alone.
MIAMI - s4story -- Developing NRX-101, an FDA-Designated Investigational Breakthrough Therapy for Suicidal Treatment-Resistant Bipolar Depression and Chronic Pain.
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on S For Story
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents. The Company believes that its current cash position will support operations into 2026 and provide sufficient capital to reach expected regulatory inflection points.
The latest NRXP key developments included the following points:
NRx Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Completion of a toxicology assessment of Benzethonium Chloride1, documenting its lack of "Generally Recognized as Safe" (GRAS) status and lack of safety data to support its use in intravenous presentations of ketamine.
NRXP filing of a Citizen's Petition with the U.S. Food and Drug Administration to seek the removal of benzethonium chloride, a toxic preservative, from all ketamine products for intravenous administration.
Filing of a patent application for NRXP NRX-100.
More on S For Story
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
HOPE Therapeutics
NRXP execution of definitive Purchase Agreement and receipt of final regulatory clearance from Florida's Agency for Health Care Administration ("ACHA") to proceed with closing the acquisition of Dura Medical.
Execution of binding letter of intent to acquire the assets of NeuroSpa TMS Holdings of Tampa, FL.
Execution of a binding letter of intent to acquire a 49% interest in Cohen and Associates, LLC.
NRXP Receipt of approval, pending legal stipulations, for $7.8 million of debt financing to support the acquisition of Dura Medical, NeuroSpa TMS Holdings, and Cohen and Associates, LLC.
Execution of a definitive purchase agreement, subject to standard closing conditions and agreement between the parties regarding the resolution of ongoing discussions, to purchase the non-clinical assets of Kadima Neuropsychiatry Institute.
Execution of a non-binding term sheet for a strategic investment from a global medical device manufacturer into HOPE.
Corporate (subsequent to the filing of form 10-Q)
NRXP $6.5 million dollar investment to purchase approximately 3.9 million shares of common stock of NRx Pharmaceuticals on August 18, 2025, by a consortium of experienced biotechnology investors led by B Group Capital. The purchase is subject to a one-year lockup on trading, shorting, or otherwise hypothecating said securities. The investment has no warrants, repricing provisions, commissions, or other structure.
The B Group Capital led consortium of ultra long-term healthcare specialist investors is highly strategic with extensive experience in complex clinical, regulatory, and commercial therapeutics but also direct ownership and management of multi-unit retail operations with potentially positive long-term implications for efforts to continue to scale and develop NRXP HOPE Therapeutics.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Designed to Help Address the Needs of Over 13 Million Americans who Seriously Consider Suicide Each Year (CDC).
FDA Fast Track Designation for NRX 100 for Suicidal Ideation in Patients with Depression, Including Bipolar Depression.
Designation Includes an FDA Determination That NRX-100 has Potential to Address an Unmet Need, Based on FDA's Assessment of Data Submitted.
13 Million Adults Seriously Consider Suicide Each Year, According to the CDC, 3.2 Million Make a Plan to Commit Suicide.
Final Clearance to Proceed to Closing of Dura Medical Acquisition from Florida's Agency for Health Care Administration (AHCA).
Actions Taken to Request the Removal of Benzethonium Chloride from Ketamine Products in Favor of the Company's Safer and Superior Options.
$7.8 Million Debt Financing to Fuel NRXP HOPE Clinic Acquisitions with
Universal Capital, LLC.
Accepted Non-Binding Potential Terms to License and Distribute NRX-100 Drug Providing Over $300 Million in Milestones Plus Tiered Double-Digit Royalties.
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) is a clinical-stage biopharmaceutical company developing therapeutics based on its NMDA platform for the treatment of central nervous system disorders, specifically suicidal bipolar depression, chronic pain and PTSD. NRXP is developing NRX-101, an FDA-designated investigational Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain
NRXP has partnered with Alvogen Pharmaceuticals around the development and marketing of NRX-101 for the treatment of suicidal bipolar depression. NRX-101 additionally has potential to act as a non-opioid treatment for chronic pain, as well as a treatment for complicated UTI.
NRXP is working on a New Drug Application for NRX-100 (IV ketamine) in the treatment of suicidal depression, based on results of well-controlled clinical trials conducted under the auspices of the US National Institutes of Health and newly obtained data from French health authorities, licensed under a data sharing agreement. NRXP was awarded Fast Track Designation for development of ketamine (NRX-100) by the US FDA as part of a protocol to treat patients with acute suicidality.
More on S For Story
- Her Magic Mushroom Memoir Launches as a Binge-Worthy Novel-to-Podcast Experience
- When You Live at the Edge of Comfort, Ordinary Days Become Defining Ones
- Century Fasteners de Mexico Hires Saúl Pedraza Gómez as Regional Sales Manager in Mexico
- Georgia Misses the Mark Again on Sports Betting, While Offshore Sites Cash In
- $40 Price Target for $NRXP in H. C. Wainright Analyst Report on Leader in $3 Billion Suicidal Depression Market with Superior NRX 100 Drug Therapy
Intravenous ketamine is widely accepted as a standard of care for acute treatment of suicidal depression, in the absence of an FDA-labeled product; the only treatment currently approved by the FDA is electroconvulsive therapy (ECT). According to the CDC, 3.8 million Americans make a plan for suicide each year.
Second Quarter 2025 Corporate Update
On August 18th NRXP announced financial results for the quarter ended June 30, 2025, and provided a corporate update. As of June 30, 2025, NRXP had approximately $2.9 million in cash and cash equivalents. The Company believes that its current cash position will support operations into 2026 and provide sufficient capital to reach expected regulatory inflection points.
The latest NRXP key developments included the following points:
NRx Drug Development
Grant of expanded Fast Track Designation for NRXP NRX-100 from the FDA for all indications and types of depression and related disorders based on its potential to satisfy an unmet medical need.
Approximately 10-fold expansion of the addressable market to 13 million Americans, compared to the original Fast Track Designation issued in 2017 for bipolar depression alone.
The Designation letter contains a specific finding that NRXP NRX-100 addresses an "unmet medical need." This is a specific qualifying requirement for the Commissioner's National Priority Voucher Program.
NRXP Filing of Commissioner's National Priority Voucher application for intravenous ketamine (NRX-100).
Submission of draft labeling for NRXP NRX-100 in the treatment of suicidal depression based on the Fast Track Designation received.
Filing of an Abbreviated New Drug Application (ANDA) for NRXP NRX-100 (preservative-free intravenous ketamine).
Submission of stability data for NRXP NRX-100 to the manufacturing data on file with FDA sufficient to support three years of room temperature shelf stability for NRX-100.
Completion of a toxicology assessment of Benzethonium Chloride1, documenting its lack of "Generally Recognized as Safe" (GRAS) status and lack of safety data to support its use in intravenous presentations of ketamine.
NRXP filing of a Citizen's Petition with the U.S. Food and Drug Administration to seek the removal of benzethonium chloride, a toxic preservative, from all ketamine products for intravenous administration.
Filing of a patent application for NRXP NRX-100.
More on S For Story
- Nashville International Chopin Piano Competition Partners with Crimson Global Academy to Support Excellence in Education
- AHRFD Releases Market Analysis: Cryptocurrency Market's Institutional Transformation Accelerating
- Dear Black Woman Anthology Releases September 20, 2025
- Ubleu Crypto Group Analyzes European Digital Asset Market Opportunities Amid Regulatory Evolution
- NIUFO Examines European MiCA Regulation's Impact on Digital Asset Trading Markets
Receipt of a PDUFA filing fee waiver from the FDA for NRXP NRX-100.
NRXP filing of module 3 manufacturing data to support a New Drug Application for NRX-101 in the treatment of patients with suicidal bipolar depression and akathisia despite treatment with already-approved medication.
HOPE Therapeutics
NRXP execution of definitive Purchase Agreement and receipt of final regulatory clearance from Florida's Agency for Health Care Administration ("ACHA") to proceed with closing the acquisition of Dura Medical.
Execution of binding letter of intent to acquire the assets of NeuroSpa TMS Holdings of Tampa, FL.
Execution of a binding letter of intent to acquire a 49% interest in Cohen and Associates, LLC.
NRXP Receipt of approval, pending legal stipulations, for $7.8 million of debt financing to support the acquisition of Dura Medical, NeuroSpa TMS Holdings, and Cohen and Associates, LLC.
Execution of a definitive purchase agreement, subject to standard closing conditions and agreement between the parties regarding the resolution of ongoing discussions, to purchase the non-clinical assets of Kadima Neuropsychiatry Institute.
Execution of a non-binding term sheet for a strategic investment from a global medical device manufacturer into HOPE.
Corporate (subsequent to the filing of form 10-Q)
NRXP $6.5 million dollar investment to purchase approximately 3.9 million shares of common stock of NRx Pharmaceuticals on August 18, 2025, by a consortium of experienced biotechnology investors led by B Group Capital. The purchase is subject to a one-year lockup on trading, shorting, or otherwise hypothecating said securities. The investment has no warrants, repricing provisions, commissions, or other structure.
The B Group Capital led consortium of ultra long-term healthcare specialist investors is highly strategic with extensive experience in complex clinical, regulatory, and commercial therapeutics but also direct ownership and management of multi-unit retail operations with potentially positive long-term implications for efforts to continue to scale and develop NRXP HOPE Therapeutics.
For more information on $NRXP visit: https://www.nrxpharma.com/ and https://compasslivemedia.com/case-study/nrx-pharmaceuticals/
Media Contact
Company Name: NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Contact Person: Matthew Duffy, Chief Business Officer
Company Website: https://www.nrxpharma.com/
Email: mduffy@nrxpharma.com
Phone: (484) 254-6134
Home Country: United States
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
Filed Under: Media
0 Comments
Latest on S For Story
- AltQuick.com Announces Continued Support for Bitcoin Testnet 3 Trading Amid Testnet 4 Launch
- Platinum Plumbing Expands Services, Rebrands as Platinum Plumbing & Water Well Services
- Voice Of Rainbow Brite, Bettina Bush Debuts First Solo Children's Album "Once Upon A Rainbow"
- Phox Health Completes 61-Minute Pharmacy-to-Patient Air Delivery Across Washington
- Dental Arts of Oklahoma Unveils New Unified Website
- Ollyball Launches Exclusive Full-Force Indoor Sports Line Nationwide at DICK'S Sporting Goods This Fall
- The Fantasy Novel of the Year: Wrath of the Spider King
- Switzer Learning Center Named Beneficiary of the 29th Annual Halloween Ball in Torrance
- New Book Empowers Job Seekers to Navigate AI-Driven Hiring: Job Hunting in the AI Era
- College Football Week 2 Odds: BookmakersReview.com Highlights Sharp Action, Line Moves, and Betting Sweats
- Heritage at South Brunswick Single Family Collection VIP opening soon!
- AI Musical Artist Kenzy Skye Releases Her Debut Hit Single "Done With You" on YouTube
- Mount Dora Frida Festival: Feel the Beat, See the Color Sat Sept 27
- Technology veteran Bill Townsend releases shocking new book about AI
- Reimagining the Literary Canon: A New Era of Authorship and Influence
- Charleston Hospitality Brings to Light the Advantage of Giving Back as a Growth Strategy
- $1.3 Billion Jackpot Fever Highlights Company Reentry to U.S. Lottery Market With Attractive New Rewards Program for AI Powered Entertainment Leader
- Revenue Optics Expands Leadership Team with Appointment of Pamela Sims as Strategic Marketing Advisor
- "Hustler's Girl Remix" Delivers a Soulful Wake-Up Call to Hustlers Neglecting Love"
- J3 Revenue Cycle Management Launches ForwardEHR Revolutionize Medical Practice Management through AI