Popular on s4story
- Libraries for Kids International Announces 2026 Board of Directors - 175
- For Valentine's Day: Treat yourself (and maybe even your sweetheart) to some Not Exactly Love Poems - 101
- Phillip E Walker's EntryLevelActing.com Actor Employment Advice E-Book Road Map Launches on MLK Day
- New Anthology Release by Dark Moon Books: HORROR LIBRARY, VOLUME 9
- 2026 Grateful American Book Prize Call for Submissions
- Author Gate Strengthens Traditional Publishing Through Investment in Writers and Editors
- Rande Vick Introduces Radical Value, Challenging How Brands Measure Long-Term Value
- Independent Comic Publisher Launches Community-Driven Anthology in South Carolina
- New Year, New Home: Begin 2026 at Heritage at South Brunswick
- Power Business Solutions Announces Joint Venture with EIG Global Trust to Deliver Data Center Financial Solutions
Similar on s4story
- Municipal Carbon Field Guide Launched by LandConnect -- New Revenue Streams for Cities Managing Vacant Land
- Aleen Inc. (C S E: ALEN.U) Advances Digital Wellness Vision with Streamlined Platform Navigation and Long-Term Growth Strategy
- RimbaMindaAI Officially Launches Version 3.0 Following Strategic Breakthrough in Malaysian Market Analysis
- Fed Rate Pause & Dow 50k: Irfan Zuyrel on Liquidity Shifts, Crypto Volatility, and the ASEAN Opportunity
- 20/20 Institute Launches Updated Vision Correction Procedures Page for Denver & Colorado Springs
- NRE-HEALTH Radio Launches With a New Approach to Health Broadcasting
- From Coffee to Commutes: sMiles App Now Pays Bitcoin for Every Gift Card Purchase
- Dr. Billy B. Laun II Addresses Over 120 Dental Professionals at Annual Dental Meeting
- CCHR: Taxpayer Billions Wasted on Mental Health Research as Outcomes Deteriorate
- Work 365 Delivers Purpose-Built Revenue Operations for Microsoft Cloud for US Government
High-Conviction CNS Disruptor Aiming to Transform Suicidal Depression, Ketamine Therapeutics, and TMS - Reaching Millions by 2030
S For Story/10679161
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) NRXP Also Reports Its Superior Preservative-Free IV Ketamine Now Submitted for FDA Abbreviated New Drug Application
MIAMI - s4story -- In a mental-health landscape where more than 13 million Americans seriously consider suicide each year, few companies are positioned as boldly—or as comprehensively—as NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP). With a newly expanded pipeline, a validated FDA regulatory path, revenue-generating clinic operations, and a third-party $34 analyst price target, NRx is surfacing as one of the most disruptive stories in central nervous system (CNS) therapeutics.
Today, the company is advancing three strategic pillars with the potential to reshape treatment for suicidal depression, chronic pain, and adjunctive neuromodulation:
Layered with manufacturing readiness, clinical-care expansion, and secured operating capital through July 2026, NRXP enters 2026 positioned for clinical, regulatory, and commercial convergence.
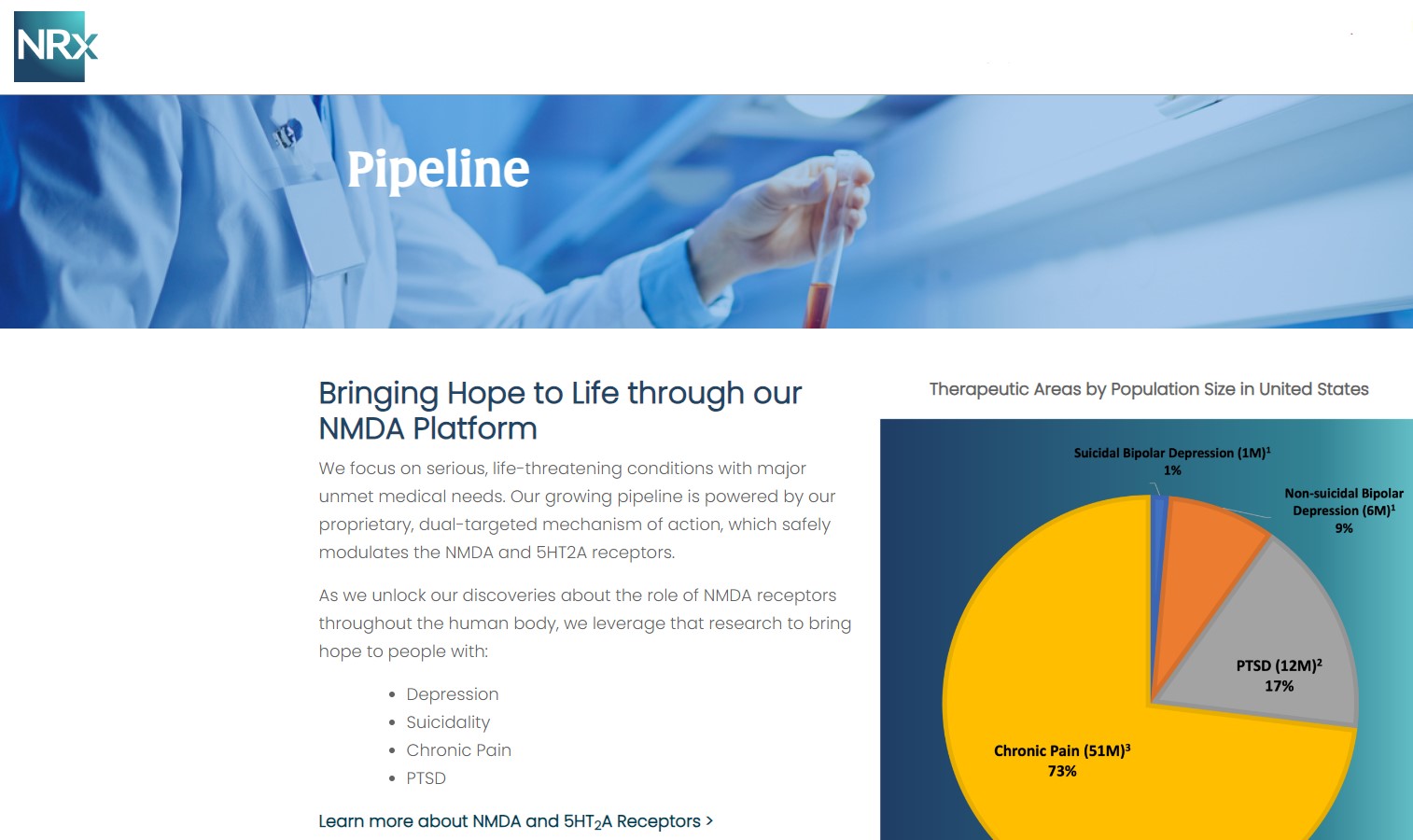
A Potential Million-Patient Market by 2030: The New NRX-101 TMS Indication
One of the most noteworthy catalysts for NRXP emerged in Q4 when the company amended its IND for NRX-101 to include use alongside Transcranial Magnetic Stimulation (TMS).
This is not incremental—it's transformational.
TMS is experiencing rapid adoption, with projections indicating over one million Americans may receive TMS annually by 2030. Yet, recent data indicate that combining TMS with NMDA-modulating therapeutics may dramatically enhance patient outcomes.
In newly presented Real World Data using a modern Theta Burst TMS device and single-day treatment protocol:
NRX-101 is uniquely positioned for this indication because:
NRXP anticipates that a confirmatory ~120-patient trial could support FDA registration for the TMS augmentation indication. Partnership discussions are already underway with TMS device manufacturers to co-develop the pivotal trial and pursue joint labeling.
More on S For Story
For investors, this opens access to a new multi-billion-dollar neuromodulation-pharma hybrid market that previously did not exist.
KETAFREE™: A Clean-Label Ketamine Positioned to Redefine a $750 Million Market
On December 2, NRXP announced a milestone with major commercial implications:
The FDA has officially received and validated the company's ANDA for KETAFREE™, its preservative-free IV ketamine formulation.
The ANDA has been deemed "substantially complete" and assigned a GDUFA goal date of July 29, 2026.
Why this matters:
1. The First Preservative-Free Ketamine in the U.S.
Most ketamine products contain benzethonium chloride (BZT)—a preservative not recognized as safe by the FDA and banned in topical antiseptics.
NRXP has filed a Citizen Petition requesting the FDA remove BZT from all U.S. ketamine products.
2. A $750M Global Generic Market Ready for Disruption
KETAFREE™ targets all existing ketamine indications with a cleaner safety profile and U.S.-based manufacturing.
The company has already manufactured initial registration lots and is prepared to scale to one million vials per month.
3. A Strategic Complement to NRX-100
KETAFREE™ follows the generic regulatory pathway (ANDA), while NRX-100 follows the innovative (NDA) pathway for suicidal depression and carries FDA Fast Track designation.
This dual-path approach not only expands the addressable market—it derisks commercialization.
NRX-100 (IV Ketamine): Fast Track, Real-World Evidence, and a Race to Fill an Unmet Clinical Void
NRXP continues rapid progress on NRX-100, an innovative ketamine-based therapy specifically developed for acute suicidal ideation.
Key elements:
This stands in contrast to Spravato®, which despite expected $1.6 billion in 2025 sales, carries labeling stating it has not been demonstrated to reduce suicidal ideation or prevent suicide.
NRXP is also seeking a Commissioner's National Priority Voucher, which could accelerate review even further.
HOPE Clinics: A Rapid-Growing Revenue Engine
More on S For Story
2025 marked NRXP's entry into active revenue generation through its HOPE Therapeutics subsidiary clinics.
This expansion supports near-term operating revenue while building a deployment platform for NRX-100 and KETAFREE™ upon approval.
Corporate Strength: Funding, Manufacturing, and Operational Execution
NRXP has accomplished several critical operational milestones:
• Cash runway secured through July 2026
Providing stability to complete pivotal regulatory steps.
• Multiple commercial drug lots manufactured
With stability data supporting three-year room-temperature shelf life.
• Real-World Data validating NRX-101's TMS synergy
Creating a new, fast-to-market indication.
• FDA Suitability Petition granted
Confirming a safe regulatory path for preservative-free ketamine.
• Analyst Price Target: $34
In an independent report by D. Boral.
Why Investors Are Paying Attention
NRx Pharmaceuticals is not a single-asset story. It is a converging portfolio of:
With suicide now a top public-health priority, NRXP is aligning itself at the crossroad of clinical need, regulatory urgency, and market expansion.
The Bottom Line
NRx Pharmaceuticals is shaping up to be one of the most compelling CNS-focused companies entering 2026. By simultaneously innovating, commercializing, and scaling, NRXP is positioning itself to influence multiple high-value markets—from TMS augmentation to suicidal depression to chronic pain and preservative-free ketamine.
As the company's clinical, regulatory, and commercial inflection points approach, NRXP is becoming one of the most closely watched emerging players in neuropsychiatry and mental-health therapeutics.
For more information:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Media Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com | Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Today, the company is advancing three strategic pillars with the potential to reshape treatment for suicidal depression, chronic pain, and adjunctive neuromodulation:
- NRX-101 – an FDA Breakthrough Therapy now targeting a newly emerging market in TMS augmentation.
- NRX-100 (IV ketamine) – an innovative therapy for acute suicidality under FDA Fast Track designation.
- KETAFREE™ – the first preservative-free IV ketamine to reach FDA ANDA review.
Layered with manufacturing readiness, clinical-care expansion, and secured operating capital through July 2026, NRXP enters 2026 positioned for clinical, regulatory, and commercial convergence.
A Potential Million-Patient Market by 2030: The New NRX-101 TMS Indication
One of the most noteworthy catalysts for NRXP emerged in Q4 when the company amended its IND for NRX-101 to include use alongside Transcranial Magnetic Stimulation (TMS).
This is not incremental—it's transformational.
TMS is experiencing rapid adoption, with projections indicating over one million Americans may receive TMS annually by 2030. Yet, recent data indicate that combining TMS with NMDA-modulating therapeutics may dramatically enhance patient outcomes.
In newly presented Real World Data using a modern Theta Burst TMS device and single-day treatment protocol:
- 87% of patients achieved clinical response
- 72% reached remission at 6 weeks
These results are particularly striking given a single administration of oral D-cycloserine, a key component of NRX-101.
NRX-101 is uniquely positioned for this indication because:
- It contains lurasidone, counteracting the low-grade hallucination risk of D-cycloserine alone.
- It holds composition-of-matter patent protection globally.
- It is an FDA Breakthrough Therapy, enabling expedited development and review.
NRXP anticipates that a confirmatory ~120-patient trial could support FDA registration for the TMS augmentation indication. Partnership discussions are already underway with TMS device manufacturers to co-develop the pivotal trial and pursue joint labeling.
More on S For Story
- Bisnar Chase Named 2026 Law Firm of the Year by Best Lawyers
- Ace Industries Welcomes Jack Polish as Controller
- Senseeker Machining Company Acquires Axis Machine to Establish Machining Capability for Improved Supply Chain Control and Shorter Delivery Times
- VC Fast Pitch Is Coming to Maryland on March 26th
- Patent Bar Exam Candidates Achieve 30% Higher Pass Rates with Wysebridge's 2026 Platform
For investors, this opens access to a new multi-billion-dollar neuromodulation-pharma hybrid market that previously did not exist.
KETAFREE™: A Clean-Label Ketamine Positioned to Redefine a $750 Million Market
On December 2, NRXP announced a milestone with major commercial implications:
The FDA has officially received and validated the company's ANDA for KETAFREE™, its preservative-free IV ketamine formulation.
The ANDA has been deemed "substantially complete" and assigned a GDUFA goal date of July 29, 2026.
Why this matters:
1. The First Preservative-Free Ketamine in the U.S.
Most ketamine products contain benzethonium chloride (BZT)—a preservative not recognized as safe by the FDA and banned in topical antiseptics.
NRXP has filed a Citizen Petition requesting the FDA remove BZT from all U.S. ketamine products.
2. A $750M Global Generic Market Ready for Disruption
KETAFREE™ targets all existing ketamine indications with a cleaner safety profile and U.S.-based manufacturing.
The company has already manufactured initial registration lots and is prepared to scale to one million vials per month.
3. A Strategic Complement to NRX-100
KETAFREE™ follows the generic regulatory pathway (ANDA), while NRX-100 follows the innovative (NDA) pathway for suicidal depression and carries FDA Fast Track designation.
This dual-path approach not only expands the addressable market—it derisks commercialization.
NRX-100 (IV Ketamine): Fast Track, Real-World Evidence, and a Race to Fill an Unmet Clinical Void
NRXP continues rapid progress on NRX-100, an innovative ketamine-based therapy specifically developed for acute suicidal ideation.
Key elements:
- Fast Track Designation from the FDA.
- An NDA expected to be completed in Q4 2025.
- Inclusion of real-world outcomes from 60,000+ IV ketamine patients, compared with 6,000 intranasal S-ketamine patients.
- Interim data from 20,000 IV ketamine patients show a faster onset and greater effect size relative to nasal S-ketamine.
This stands in contrast to Spravato®, which despite expected $1.6 billion in 2025 sales, carries labeling stating it has not been demonstrated to reduce suicidal ideation or prevent suicide.
NRXP is also seeking a Commissioner's National Priority Voucher, which could accelerate review even further.
HOPE Clinics: A Rapid-Growing Revenue Engine
More on S For Story
- Bestselling Author and Poet Uplifts Readers with Reflections on Faith, Resilience & Social Justice
- Municipal Carbon Field Guide Launched by LandConnect -- New Revenue Streams for Cities Managing Vacant Land
- Hoy Law Wins Supreme Court Decision Establishing Federal Trucking Regulations as the Standard of Care in South Dakota
- Dr. Rashad Richey's Indisputable Shatters Records, Over 1 Billion YouTube Views, Top 1% Podcast, 3.2 Million Viewers Daily
- Maya Christobel Releases New Book - The Third State of Love
2025 marked NRXP's entry into active revenue generation through its HOPE Therapeutics subsidiary clinics.
- Three facilities currently operating in Florida.
- Three additional locations expected by year-end.
- Focus includes depression, PTSD, interventional psychiatry, military and first-responder mental-health care.
This expansion supports near-term operating revenue while building a deployment platform for NRX-100 and KETAFREE™ upon approval.
Corporate Strength: Funding, Manufacturing, and Operational Execution
NRXP has accomplished several critical operational milestones:
• Cash runway secured through July 2026
Providing stability to complete pivotal regulatory steps.
• Multiple commercial drug lots manufactured
With stability data supporting three-year room-temperature shelf life.
• Real-World Data validating NRX-101's TMS synergy
Creating a new, fast-to-market indication.
• FDA Suitability Petition granted
Confirming a safe regulatory path for preservative-free ketamine.
• Analyst Price Target: $34
In an independent report by D. Boral.
Why Investors Are Paying Attention
NRx Pharmaceuticals is not a single-asset story. It is a converging portfolio of:
- Breakthrough-designated CNS drugs
- A first-in-class preservative-free ketamine
- A new TMS-augmentation market with million-patient potential
- A scalable manufacturing base
- A growing national clinical footprint
- A strategic partnership with Alvogen
- Fast Track regulatory momentum
With suicide now a top public-health priority, NRXP is aligning itself at the crossroad of clinical need, regulatory urgency, and market expansion.
The Bottom Line
NRx Pharmaceuticals is shaping up to be one of the most compelling CNS-focused companies entering 2026. By simultaneously innovating, commercializing, and scaling, NRXP is positioning itself to influence multiple high-value markets—from TMS augmentation to suicidal depression to chronic pain and preservative-free ketamine.
As the company's clinical, regulatory, and commercial inflection points approach, NRXP is becoming one of the most closely watched emerging players in neuropsychiatry and mental-health therapeutics.
For more information:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
Website: www.nrxpharma.com
Media Contact: Matthew Duffy, Chief Business Officer
Email: mduffy@nrxpharma.com | Phone: (484) 254-6134
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: CorporateAds
0 Comments
Latest on S For Story
- Northwest Modern Fabrication Expands Manufacturing Capacity With 4,800 Sq. Ft. Addition
- People & Stories/Gente y Cuentos to Host Award-Winning Novelist Susan Choi for Spring Literary Benefit Event
- NRE-HEALTH Radio Launches With a New Approach to Health Broadcasting
- From Coffee to Commutes: sMiles App Now Pays Bitcoin for Every Gift Card Purchase
- Finland's Health Authority Launches '2-4-2' Gambling Risk Limits Ahead of Expected Advertising Boom
- [New Book] "How Forest Park Was Made" Available Now!
- Dr. Billy B. Laun II Addresses Over 120 Dental Professionals at Annual Dental Meeting
- CCHR: Taxpayer Billions Wasted on Mental Health Research as Outcomes Deteriorate
- Digital Efficiency Consulting Group (DECG) Officially Launches
- New Book Explores the Rich History of Hiking
- Work 365 Delivers Purpose-Built Revenue Operations for Microsoft Cloud for US Government
- Meridianvale Unveils QarvioFin Public Beta: The First 'Glass Box' AI Operating System for Autonomous Finance
- Mend Colorado Launches Revamped Sports Performance Training Page
- Authoress S.E. Gregg Offers Gold-Signed Copies in 2026"
- "They Thought It Was Impossible to Expose Them — This Is Exactly How It Was Done"
- Love Against Oblivion: Uri J. Nachimson's KADOSH
- Raconteur Press Announces Promotions, Increased Focus on Boys Adventure Books
- Parkway Prosthodontics Achieves Breakthrough Full-Arch Reconstruction Case
- Postmortem Pathology Expands to Phoenix: Bringing Families Answers During Their Most Difficult Moments
- Blasting Off with Space Sector Companies: Artemis II Manned Moon Mission is Set to Launch: Could $ASTI be on the Same Rocket Ride as $ASTS & $LUNR?