Popular on s4story
- Military Writers Society of America Announces 2025 Awards Season Medalists - 135
- Iguabit Unveils Comprehensive Platform Strategy for Brazilian Crypto Traders Seeking Regulated Solutions - 132
- Southland Symphony Orchestra Season Opener – A Musical Mosaic - 123
- Planting People Growing Justice™ Leadership Institute Honors Nine Leaders with Awards - 122
- AdvisorVault Releases New Explainer Video on their 17a-4 Managed 365 Service - 119
- RNHA FL Unveils Bold New Leadership Ahead of 2026 Elections - 116
- Imprint Showcase: Nocturnum's Muse – Horror with Heart - 114
- Mullins McLeod Surges Into SC Governor's Race with $1.4 Million Raised in First Quarter; Most from His Own Commitment, Not Political Pockets - 112
- World Premiere English translation of Rilke's Testament - 102
- Planting People Growing Justice Leadership Institute Announces 2025 Children's Book Award Winners - 101
Similar on s4story
- Kaltra unveils reversible microchannel coils – engineered for modern heat pumps
- Phinge Announces Proposal to Combat Billions in Government Waste, Fraud, and Abuse with Proactive, Hardware-Verified Netverse App-Less Platform
- Taboo: The Lost Codes of Men — A Bold New Book Confronting the Crisis of Modern Manhood
- Phinge's Netverse to Redefine Clinical Trial Safety and Data Integrity with Netverse Patented, Hardware-Verified Platform
- 'Wild Hermit Wellness' Has Achieved Bestseller Status in Just 2 Months Since Launch Of Organic Skincare Line
- Poised for Major Growth with Strategic Military Orders, Global Expansion, and Groundbreaking Underground Mining Initiative $RMXI
- Mensa Foundation's New Science Program Encourages Hands-On Discovery
- Golden Paper Introduces TAD Hand Towel Technology, Ushering in a New Era of Premium Tissue Quality
- OfficeSpaces.co Expands Its AI-Powered Website Builder Across North America
- Why Generic Platforms Fail in Emerging Markets: Bettorify Exposes the Gap Between Promise and Reality
Lineus Medical Obtains CE Mark for Flagship Product SafeBreak Vascular
S For Story/10673546
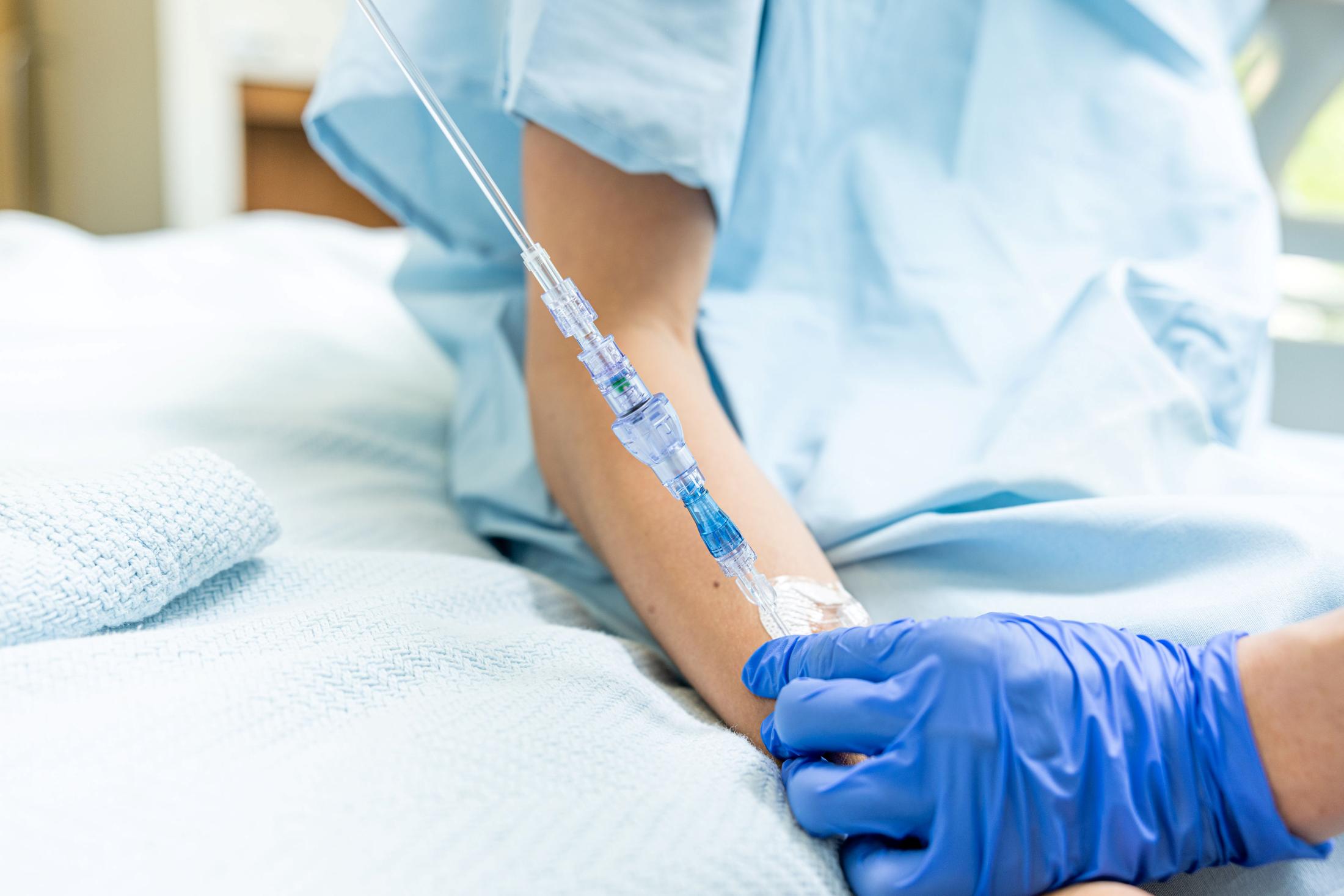
FAYETTEVILLE, Ark. - s4story -- Lineus Medical has obtained CE Mark certification for its flagship product, SafeBreak® Vascular, the only breakaway device for IV lines clinically proven to reduce multiple IV complications.1 With this certification, SafeBreak Vascular is now cleared for use across the European Union. This approval expands SafeBreak Vascular's clearance from 5 countries to 32, spanning across more than three continents. Lineus Medical is actively partnering with distributors across Europe to expand hospital access to SafeBreak worldwide.
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on S For Story
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
SafeBreak Vascular is a break-away device for IV lines clinically proven to reduce IV complications1. When a harmful force is placed on the line, SafeBreak intentionally separates to remove the damaging force and protect the patient's IV. When SafeBreak separates, valves on both ends of the device close preventing medicine spills from the pump and blood loss from the patient. To replace SafeBreak, each component is unthreaded from the line, a new sterile SafeBreak is installed, and the patent's infusion is restarted. Patients avoid additional needlesticks, nurses save time, and hospitals save money1.
More on S For Story
- Author Calls Trump the Most Racist President in US History
- New Book "Man-Interrupted" Exposes the Ongoing Attack on Black Masculinity
- Michelle L Crocker releases a new book called Do Not Date An Asshole
- Taboo: The Lost Codes of Men — A Bold New Book Confronting the Crisis of Modern Manhood
- Phinge's Netverse to Redefine Clinical Trial Safety and Data Integrity with Netverse Patented, Hardware-Verified Platform
"Earning the CE Mark is a major milestone in our company's journey," says Vance Clement, CEO of Lineus Medical. "It reflects our commitment to remove the pains associated with IV lines and make SafeBreak Vascular the standard of care worldwide. Our goal is to be the leading company in this market segment in both science and sales. This clearance affirms that position and makes SafeBreak the first break-away device to have clearance in Asia, Europe, and North America."
Larry Hayes, VP of Sales comments, "With CE Mark approval, we are opening the door to new distribution partnerships across Europe. This certification allows SafeBreak Vascular to reach more hospitals and healthcare systems, accelerating our global commercialization strategy and expanding our global footprint."
"Achieving CE Mark certification is a testament to the quality and reliability of both our product and our manufacturing processes," said Will Armstrong, COO. "It reflects the strength of our team and our commitment to maintaining the highest standards."
About Lineus Medical
Lineus Medical is the developer of SafeBreak Vascular, a breakaway technology proven to reduce IV restarts and IV complications.1 Our mission is to remove the pains associated with medical lines. More information about Lineus Medical can be found at www.lineusmed.com. Follow Lineus Medical on LinkedIn, Facebook, and Instagram.
References:
1. Data on File
Source: Lineus Medical
0 Comments
Latest on S For Story
- Poised for Major Growth with Strategic Military Orders, Global Expansion, and Groundbreaking Underground Mining Initiative $RMXI
- New Children's Picture Book Celebrates Lao American Family, Food & Heritage
- XRP fever is coming again, WOA Crypto helps the new trend and earns tens of thousands of dollars a day
- Dr. Simon Poole and Chef Amy Riolo Release a Groundbreaking Guide to Managing Cholesterol
- Inflation Rebounds Under Tariff Shadow: Wall Street Veteran Kieran Winterbourne Says Macro Signals Matter More Than Market Sentiment
- Bruce Goldwell Launches "The Silver Brain" Helping Seniors Stay Sharp, Happy, and Young
- Mensa Foundation's New Science Program Encourages Hands-On Discovery
- Golden Paper Introduces TAD Hand Towel Technology, Ushering in a New Era of Premium Tissue Quality
- ReedSmith® Creates Founder-Investor Connections at The Investor Dating Game™ by Tech Coast Venture Network During LA Tech Week
- OfficeSpaces.co Expands Its AI-Powered Website Builder Across North America
- Tobu Railway Group Will Host the Fourth Annual "Take-Akari" Bamboo Lantern Festival in East Tokyo, November 7, 2025 – January 31, 2026
- New Article by Roy J. Meidinger – Examines Hidden Hidden Healthcare Kickbacks
- Why Generic Platforms Fail in Emerging Markets: Bettorify Exposes the Gap Between Promise and Reality
- Children's Book Author Launches 'Karma Cats Puppy Quest' to Champion Helping Lost Pets
- Blogging Pioneer Sherry Bennett Celebrates 29 Years Online - Sharing the Secrets Behind Her 7-Figure Blog Empire
- Koplon Dentistry Elevates Implant Expertise with Advanced CE Course
- i2 Group Acquisitions and Investments in Innovations Deliver 40% Increase in Year-on-Year Bookings
- New Book Release: The Tree That Could Not Change
- BayWa r.e. Solar Trade and WHES Announce Distribution Partnership for the European Market: Delivering Smarter Energy Storage
- Fleet Mining Cloud Mining Platform — Latest Guide: Making Bitcoin Mining Safer and More Convenient