Popular on s4story
- OneVizion Announces Next Phase of Growth as Brad Kitchens Joins Board of Directors - 155
- Ice Melts. Infrastructure Fails. What Happens to Clean Water? - 139
- Donna L. Quesinberry, President of DonnaInk Publications, Unveils New Article on Author Monetization - 129
- Still Using Ice? FrostSkin Reinvents Hydration - 126
- "They Thought It Was Impossible to Expose Them — This Is Exactly How It Was Done"
- Authoress S.E. Gregg Offers Gold-Signed Copies in 2026"
- Mend Colorado Launches Revamped Sports Performance Training Page
- New Children's Picture Book "Diwa of Mount Luntian" Focuses on Calm, Culture, and Connection for Today's Families
- Work 365 Delivers Purpose-Built Revenue Operations for Microsoft Cloud for US Government
- Cold. Clean. Anywhere. Meet FrostSkin
Similar on s4story
- National Expansion Ignited Across Amazon $AMZN, Chewy $CHWY & Walmart $WMT: NDT Pharmaceuticals, Inc. (Stock Symbol: NDTP) $NDTP
- Tarrytown Expocare Pharmacy Announces Strategic Leadership Appointments to Accelerate Growth and Innovation
- NaturismRE Launches the NRE Health Institute to Advance Evidence-Informed Public Health Research
- Progressive Dental & The Closing Institute Partner with Zest Dental Solutions to Elevate Full-Arch Growth and Patient Outcomes
- CCHR: While Damaging Antipsychotics Win Approval, Proven Non-Drug Alternatives Remain Ignored
- At 25, She Became One of the Youngest AAPI Female Founders to Win One of the World's Most Prestigious Design Awards for a Lamp That Makes You Smile
- VENUS Goes Live on CATEX Exchange As UK Financial Ltd Activates The Premier Division Of The Maya Meme's League
- Special Alert: Undervalued Opportunity: IQSTEL (N A S D A Q: IQST) Positioned for Explosive Multi-Year Growth
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
- Vesica Health Receives FDA Breakthrough Device Designation for AssureMDx
$750 Million Market on Track to $3.35 Billion by 2034: $NRXP Launches First-in-Florida "One Day" Depression Treatment in Partnership with Ampa Health
S For Story/10677276
Analyst D. Boral Targets NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP) $NRXP at $34 Per Share — Pioneering Breakthroughs in Treatment-Resistant Depression and Chronic Pain
MIAMI - s4story -- NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP), a clinical-stage biopharmaceutical innovator developing breakthrough therapeutics for central nervous system disorders, has emerged as a potential leader in the next generation of psychiatric care. With the global ketamine market projected to expand from $750 million to $3.35 billion by 2034, NRXP is strategically positioned to capture a substantial share through a suite of FDA-designated and investigational treatments aimed at addressing urgent mental health needs.
Revolutionary "ONE-D" Depression Treatment Launches in Florida
NRx recently announced the first-in-Florida launch of its "One Day" (ONE-D) depression treatment, developed in partnership with Ampa Health. The ONE-D protocol uses Ampa's advanced Transcranial Magnetic Stimulation (TMS) device — the first reported technology capable of achieving remission from treatment-resistant depression in a single day.
Unlike conventional 90-day TMS regimens, the ONE-D treatment integrates a single day of precision TMS with D-cycloserine and lisdexamfetamine (both utilized under physician supervision), achieving up to 87% response and 72% remission rates in peer-reviewed nonrandomized studies.
The technology is initially deployed at multiple NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, with expansion to six Florida locations by year-end 2025 under the direction of Dr. Rebecca Cohen, Medical Director of HOPE Clinics.
More on S For Story
NRX-101: A Breakthrough Therapy for Suicidal Bipolar Depression
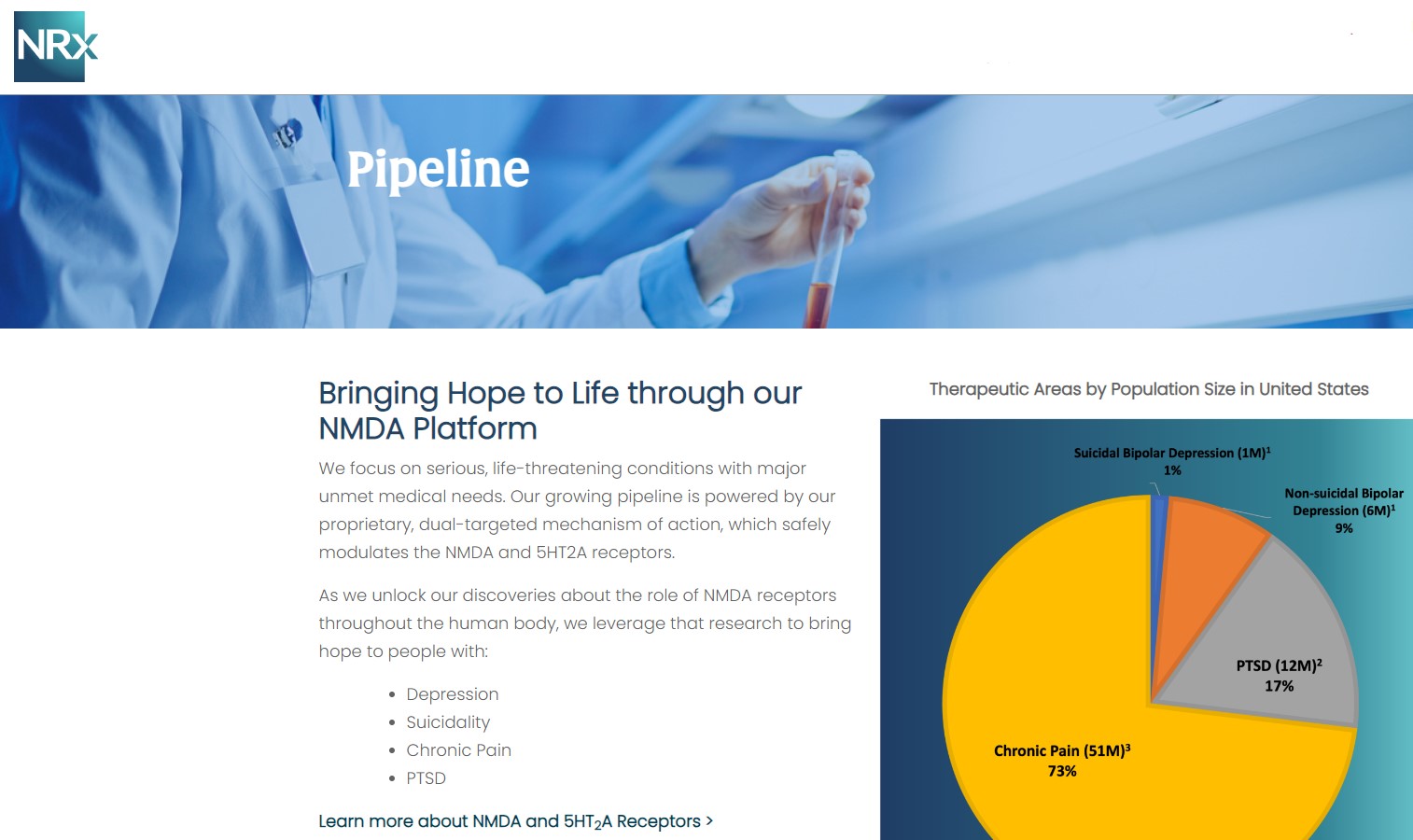
Central to NRx's pipeline is NRX-101, an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. The therapy leverages the company's proprietary NMDA platform and is being developed in partnership with Alvogen Pharmaceuticals.
NRX-101 also holds potential as a non-opioid pain treatment and for other indications such as complicated urinary tract infections (UTIs). With over 13 million Americans seriously considering suicide each year (CDC), NRXP's drug candidates could address one of the nation's most pressing health crises.
KETAFREE™: FDA-Focused Push to Modernize Ketamine Treatment
NRXP recently re-filed its Abbreviated New Drug Application (ANDA) for KETAFREE™, a preservative-free IV ketamine formulation, after securing FDA approval of its Suitability Petition. By eliminating benzethonium chloride (BZT) — a preservative linked to neurotoxicity — KETAFREE™ represents a safer, next-generation alternative to traditional ketamine products.
The company has also petitioned the FDA to formally remove BZT from IV formulations, citing expert toxicological data and the growing demand for preservative-free therapies in modern medicine. Given the ongoing ketamine shortage reported by the American Society of Hospital Pharmacists, NRXP's KETAFREE™ could fill a critical supply gap and strengthen its market positioning.
Strategic Acquisitions and Growth Initiatives
NRXP recently completed the acquisition of Dura Medical, an EBITDA-positive network of interventional psychiatry clinics delivering advanced therapies for depression and PTSD. This acquisition, along with pending deals for Neurospa TMS and Cohen & Associates, expands NRXP's reach to more than eight Florida clinic locations, reinforcing its vertically integrated mental health care model.
More on S For Story
To accelerate this rollout, NRXP secured $7.8 million in debt financing from Universal Capital, LLC to fund further HOPE Clinic acquisitions.
Additionally, NRXP has accepted non-binding licensing terms for NRX-100, a drug candidate expected to generate over $300 million in potential milestone payments and tiered double-digit royalties upon commercialization.
Strong Analyst Endorsement
In a recently released report, D. Boral Research issued a Buy rating on NRXP with a $34 price target, citing the company's expanding clinical pipeline, growing footprint in the interventional psychiatry sector, and first-mover advantage in the transformative ONE-D treatment platform.
A Vision for Transforming Mental Health
From its FDA Fast Track designation for IV ketamine (NRX-100) to its breakthrough therapy designation for NRX-101, NRx Pharmaceuticals continues to redefine the boundaries of psychiatric medicine. Its commitment to science-driven innovation — now coupled with real-world clinic deployment through HOPE Clinics — positions NRXP as both a therapeutic pioneer and a potentially lucrative opportunity in the rapidly expanding mental health market.
Company Contact:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
📍 Wilmington, DE
Matthew Duffy, Chief Business Officer
📧 mduffy@nrxpharma.com | ☎️ (484) 254-6134
🌐 www.nrxpharma.com
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Revolutionary "ONE-D" Depression Treatment Launches in Florida
NRx recently announced the first-in-Florida launch of its "One Day" (ONE-D) depression treatment, developed in partnership with Ampa Health. The ONE-D protocol uses Ampa's advanced Transcranial Magnetic Stimulation (TMS) device — the first reported technology capable of achieving remission from treatment-resistant depression in a single day.
Unlike conventional 90-day TMS regimens, the ONE-D treatment integrates a single day of precision TMS with D-cycloserine and lisdexamfetamine (both utilized under physician supervision), achieving up to 87% response and 72% remission rates in peer-reviewed nonrandomized studies.
The technology is initially deployed at multiple NRXP HOPE Clinics in Sarasota, Naples, and Fort Myers, with expansion to six Florida locations by year-end 2025 under the direction of Dr. Rebecca Cohen, Medical Director of HOPE Clinics.
More on S For Story
- Summit Appoints Javier Cabeza as Data, AI, and Analytics Practice Lead
- March Is Skiing's Smartest Buying Window
- Cancun Airport Transportation Expands Fleet Ahead of Record Passenger Growth at Cancun International Airport
- Tobu Group's "T-home Series" of Accommodations in Tokyo Just Opened "T-home KEI."
- Custom Wooden Token Manufacturer Celebrates 10 Years of Helping Brands Stay Top of Mind
NRX-101: A Breakthrough Therapy for Suicidal Bipolar Depression
Central to NRx's pipeline is NRX-101, an FDA-designated Breakthrough Therapy for suicidal treatment-resistant bipolar depression and chronic pain. The therapy leverages the company's proprietary NMDA platform and is being developed in partnership with Alvogen Pharmaceuticals.
NRX-101 also holds potential as a non-opioid pain treatment and for other indications such as complicated urinary tract infections (UTIs). With over 13 million Americans seriously considering suicide each year (CDC), NRXP's drug candidates could address one of the nation's most pressing health crises.
KETAFREE™: FDA-Focused Push to Modernize Ketamine Treatment
NRXP recently re-filed its Abbreviated New Drug Application (ANDA) for KETAFREE™, a preservative-free IV ketamine formulation, after securing FDA approval of its Suitability Petition. By eliminating benzethonium chloride (BZT) — a preservative linked to neurotoxicity — KETAFREE™ represents a safer, next-generation alternative to traditional ketamine products.
The company has also petitioned the FDA to formally remove BZT from IV formulations, citing expert toxicological data and the growing demand for preservative-free therapies in modern medicine. Given the ongoing ketamine shortage reported by the American Society of Hospital Pharmacists, NRXP's KETAFREE™ could fill a critical supply gap and strengthen its market positioning.
Strategic Acquisitions and Growth Initiatives
NRXP recently completed the acquisition of Dura Medical, an EBITDA-positive network of interventional psychiatry clinics delivering advanced therapies for depression and PTSD. This acquisition, along with pending deals for Neurospa TMS and Cohen & Associates, expands NRXP's reach to more than eight Florida clinic locations, reinforcing its vertically integrated mental health care model.
More on S For Story
- NaturismRE Launches the NRE Health Institute to Advance Evidence-Informed Public Health Research
- P-Wave Classics to publish Robert Bage's Hermsprong in three volumes, beginning 12 May
- Progressive Dental & The Closing Institute Partner with Zest Dental Solutions to Elevate Full-Arch Growth and Patient Outcomes
- CCHR: While Damaging Antipsychotics Win Approval, Proven Non-Drug Alternatives Remain Ignored
- Arcuri Group Announces Long‑Term Partnership with WakeMed Health & Hospitals to Deliver Situational Awareness and De‑escalation Training
To accelerate this rollout, NRXP secured $7.8 million in debt financing from Universal Capital, LLC to fund further HOPE Clinic acquisitions.
Additionally, NRXP has accepted non-binding licensing terms for NRX-100, a drug candidate expected to generate over $300 million in potential milestone payments and tiered double-digit royalties upon commercialization.
Strong Analyst Endorsement
In a recently released report, D. Boral Research issued a Buy rating on NRXP with a $34 price target, citing the company's expanding clinical pipeline, growing footprint in the interventional psychiatry sector, and first-mover advantage in the transformative ONE-D treatment platform.
A Vision for Transforming Mental Health
From its FDA Fast Track designation for IV ketamine (NRX-100) to its breakthrough therapy designation for NRX-101, NRx Pharmaceuticals continues to redefine the boundaries of psychiatric medicine. Its commitment to science-driven innovation — now coupled with real-world clinic deployment through HOPE Clinics — positions NRXP as both a therapeutic pioneer and a potentially lucrative opportunity in the rapidly expanding mental health market.
Company Contact:
NRx Pharmaceuticals, Inc. (N A S D A Q: NRXP)
📍 Wilmington, DE
Matthew Duffy, Chief Business Officer
📧 mduffy@nrxpharma.com | ☎️ (484) 254-6134
🌐 www.nrxpharma.com
DISCLAIMER: https://corporateads.com/disclaimer/
Disclosure listed on the CorporateAds website
Source: Corporate Ads
0 Comments
Latest on S For Story
- Primeindexer Google indexing platform launched by SEO Danmark APS
- Kaltra Introduces New Downward-Spraying Distribution Technology to Boost Microchannel Evaporator Performance
- Talentica Announces Winners of Multi-Agent Hackathon 2026
- DonnaInk Publications Announces 2026 2nd Ed. Releases of Two Signature Series by Dr. Gerhard
- Steven Kay's Deceptive Enticements Titles Expand Into Mass Market With New Foreign Publicity Deal
- Cape Cod Author and Coast Guard Veteran Breaks Silence on PTSD in New Memoir 'Hold Fast'
- Award-Winning Author & TEDx Speaker Reggie D. Ford Releases New Children's Book
- Special Alert: Undervalued Opportunity: IQSTEL (N A S D A Q: IQST) Positioned for Explosive Multi-Year Growth
- Triple-Digit Growth, Strategic N A S D A Q Uplist, Plus A Scalable Healthcare Rollout Model: Stock Symbol: CDIX
- Vesica Health Receives FDA Breakthrough Device Designation for AssureMDx
- Michael Roberts Announces the release of Ink Magic: Legacy of the Flower
- Lineus Medical's SafeBreak® Vascular Added to Alliant GPO Contract
- Gyanendra Rana Releases New Self-Help Book - A Monkey Became a Monk
- Cancun All Inclusive is ready for Spring Break 2026 with new Resorts, Exclusive Deals, activities and more!
- 66% of US Bankruptcies Are Medical — So Americans Are Building Businesses That Cover Healthcare Emergencies
- DonnaInk Launches 2026 Author Spotlight Initiative to Elevate Independent Voices
- Husband and Wife Release Children's Book and Donate Proceeds to Non-Profit
- Ludex Partners With Certified Trading Card Association (CTCA) To Elevate Standards And Innovation In The Trading Card Industry
- Digi 995: The War of Eldoria Expands the Sci-Fi Saga in Explosive Book Two
- Dante King Releases The Psychopathy of Whiteness, A Groundbreaking Examination of Racism